Chem 51A 10/05/09 Ch. 1. Molecular Geometry. Ch. 2. Acids and Bases
TLDRThis lecture delves into molecular geometry, focusing on sp3, sp2, and sp hybridized atoms, and their implications on the structure of organic molecules like methane, ammonia, and water. It transitions to acids and bases, introducing the concept of curved arrow notation crucial for visualizing electron flow in reactions. The Bronsted-Lowry theory is discussed, illustrating how acids donate protons and bases accept them, with examples including HCl, methanol, and methyl ammonium ion reactions. The lecture concludes with an introduction to PKA, emphasizing the importance of understanding acid strength in organic chemistry.
Takeaways
- 📚 The lecture concludes chapter one and transitions into chapter two, focusing on molecular geometry and the organic chemistry of acids and bases.
- 🧬 The concept of molecular geometry is revisited, specifically sp3 hybridization, which results in a tetrahedral arrangement with bond angles of approximately 109°.
- 🌐 The lecture explains how the presence of lone pairs affects the bond angles in molecules like ammonia (NH3) and water (H2O), causing deviations from the ideal tetrahedral angle.
- 🔬 VPR (Valence Shell Electron Pair Repulsion) Theory is discussed as a fundamental concept in understanding molecular geometry in organic chemistry.
- 🔍 The script delves into sp2 hybridized atoms, which have bond angles of about 120° and are exemplified by molecules like ethene and the methyl carbocation.
- 🌀 The s hybridization is introduced, characterized by linear arrangements and bond angles of 180°, as seen in molecules like acetylene and acetonitrile.
- 🔑 Curved arrow notation is highlighted as a central concept in organic chemistry, essential for depicting the flow of electrons during reactions.
- 🌾 The lecture connects general chemistry concepts of acids and bases to organic chemistry, emphasizing their role in generating reactive species like electrophiles and nucleophiles.
- ⚗️ Bronsted-Lowry theory defines acids as proton donors and bases as proton acceptors, with examples provided to illustrate the formation of conjugate acids and bases.
- 🔄 The mechanism of acid-base reactions is illustrated using curved arrows to show the transfer of protons and the resulting changes in molecular structure.
- 📉 The script introduces the concept of PKA, which measures the strength of an acid in solution, and explains its significance in comparing the dissociation of different acids.
Q & A
What is the main focus of the lecture script provided?
-The lecture script focuses on molecular geometry, particularly sp3, sp2, and sp hybridization, and transitions into discussing acids and bases in the context of organic chemistry, including their relationship to reactivity and the use of curved arrow notation.
What is the significance of the tetrahedral arrangement in sp3 hybridized atoms?
-The tetrahedral arrangement in sp3 hybridized atoms, with bond angles of approximately 109°, is significant because it describes the spatial orientation of the four ligands or substituents around the central atom, as seen in molecules like methane.
How does the presence of a lone pair of electrons affect the bond angles in ammonia (NH3)?
-The presence of a lone pair of electrons in ammonia causes the bond angles to be slightly less than the ideal tetrahedral angle of 109°, resulting in a bond angle of approximately 107.3° due to the lone pair taking up more space than bonding pairs.
What is the molecular geometry of water (H2O) and how does it differ from the ideal sp3 hybridization?
-Water has a bent molecular geometry with a bond angle of approximately 104.5°. This is a deviation from the ideal tetrahedral angle due to the presence of two lone pairs on the oxygen atom, which take up more space and compress the H-O-H bond angle.
What is the relationship between the VSEPR theory and the molecular geometries discussed in the script?
-The Valence Shell Electron Pair Repulsion (VSEPR) theory is used to predict molecular geometries based on the repulsion between electron pairs in the valence shell of an atom. The script discusses how this theory applies to sp3, sp2, and sp hybridized atoms to explain the geometries of molecules like methane, ammonia, and water.
What is the significance of the term 'curved arrow notation' in organic chemistry?
-Curved arrow notation is a method used in organic chemistry to represent the movement of electron pairs during chemical reactions, helping to visualize the formation and breaking of bonds, which is central to understanding reactivity in organic molecules.
How does the script relate the concept of acids and bases in general chemistry to organic chemistry?
-The script relates the concept by explaining that Bronsted-Lowry acids and bases, which are proton donors and acceptors respectively, have parallels in organic chemistry as electrophiles and nucleophiles, which are species that accept and donate electrons during reactions.
What is the definition of a Bronsted-Lowry acid according to the script?
-A Bronsted-Lowry acid is defined as a substance that donates a proton (H+) in a chemical reaction, which is a fundamental concept in both general and organic chemistry.
Can you provide an example of how curved arrow notation is used to illustrate an acid-base reaction from the script?
-An example from the script is the reaction between water and hydrogen chloride (HCl), where the lone pair of electrons on the oxygen atom in water is used to form a bond with the proton from HCl, resulting in the formation of hydronium ion (H3O+) and chloride ion (Cl-).
What is the PKA and how does it relate to the strength of an acid?
-PKA is the negative logarithm of the acid dissociation constant (Ka). It is used to measure the degree of acidity, with lower PKA values indicating stronger acids because they dissociate more in water to form hydronium ions.
How does the script explain the concept of 'free base' in the context of organic chemistry?
-The script explains the concept of 'free base' by describing the reaction of an amine salt, such as amine hydrochloride, with a base like sodium hydroxide to remove the proton from the ammonium ion, generating the free amine, which is the physiologically active form.
Outlines
📚 Introduction to Molecular Geometry and Acid-Base Chemistry
The speaker begins by wrapping up concepts from chapter one on molecular geometry, specifically focusing on sp3 hybridization and its implications for the structure of molecules like methane. They then transition into chapter two, which delves into acids and bases, their relationship to organic chemistry, and the importance of curved arrow notation. The summary emphasizes the connection between general chemistry concepts and their specific applications in organic chemistry, setting the stage for a deeper exploration of reactivity.
🔍 Exploring Molecular Geometries: From Tetrahedral to Bent
This paragraph delves into the molecular geometries of various compounds, starting with sp3 hybridized atoms like methane and moving through ammonia (NH3), water (H2O), and other molecules with lone pairs and bonding pairs. The summary explains how the presence of lone pairs affects bond angles, causing deviations from the ideal tetrahedral angle of 109°, and introduces the concept of VPR (Valence Shell Electron Pair Repulsion) Theory. It also touches on the transition from inorganic molecules like water to organic molecules like methanol, maintaining the same geometries.
📐 SP2 Hybridization and the Planar Geometry of Ethene
The speaker discusses SP2 hybridization, which results in a planar trigonal arrangement with bond angles of approximately 120°. Using ethene as an example, the summary explains the orbital-based model of its structure, highlighting the planarity and the significance of the double bond. It also mentions the methyl carbocation, emphasizing its planar trigonal shape, the presence of a vacant p orbital, and its instability, which is rarely seen in reactions without being stabilized by other groups.
🌟 Linear Geometry of SP Hybridized Atoms in Acetylene
This paragraph introduces SP hybridization, characterized by linear arrangements with 180° bond angles. The summary covers examples like acetylene, where the carbon atoms are SP hybridized, resulting in a linear geometry. It also introduces acetonitrile (CH3CN) and allene, noting their unique geometries and the impact of substituents on bond angles, especially when lone pairs are present.
🌱 Transitioning to Acids and Bases in Organic Chemistry
The speaker shifts the focus to chapter two, discussing the central role of acids and bases in organic chemistry. The summary outlines the importance of these species in generating reactive entities like electrophiles and nucleophiles and introduces the concept of curved arrow notation. It sets the stage for understanding the mechanisms of acid-base reactions and their significance in organic reactivity.
🔑 The Bronsted-Lowry Definition of Acids and Bases
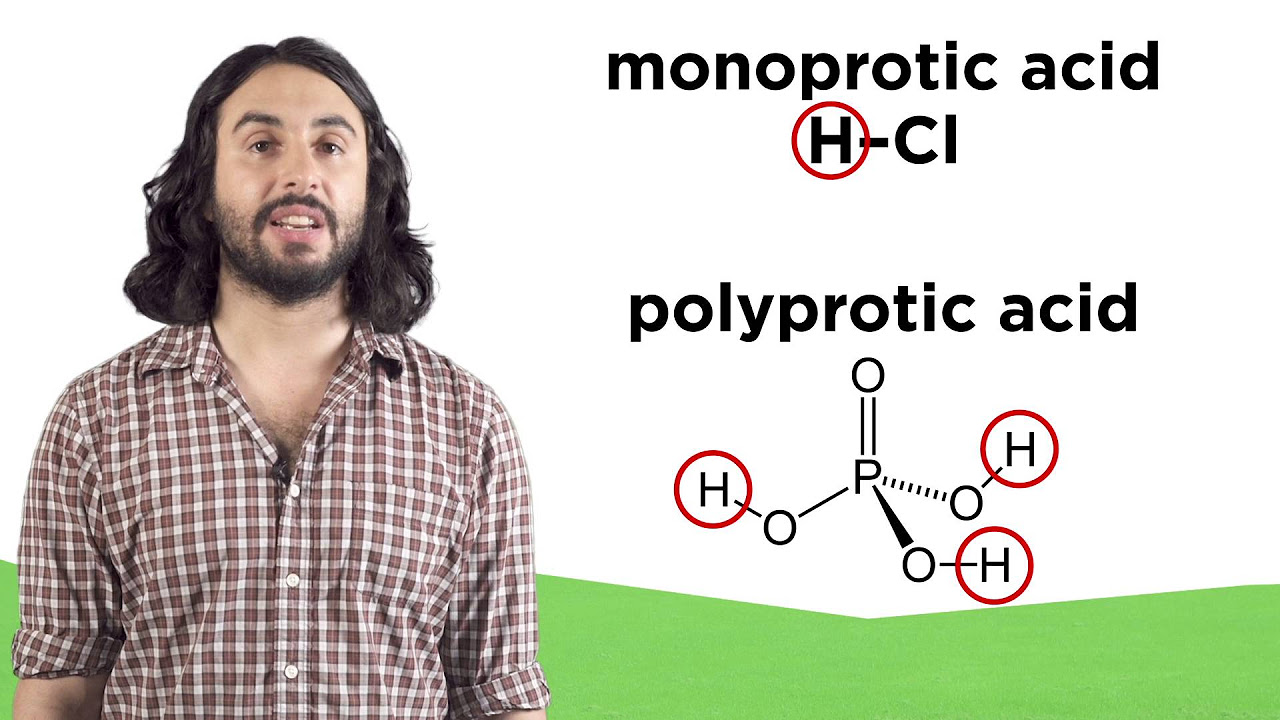
This paragraph introduces the Bronsted-Lowry definition of acids and bases, with acids being proton donors and bases being proton acceptors. The summary explains the concept of conjugate acids and bases, using HCl in water as an example to illustrate the formation of H3O+ and Cl-. It also discusses the use of curved arrow notation to depict the flow of electrons in these reactions, providing a visual representation of bond formation and breaking.
🚀 Acid-Base Reactions with Organic Compounds
The speaker extends the discussion of acid-base reactions to organic compounds, using methanol and the ammonium ion as examples. The summary describes how these reactions parallel those in inorganic chemistry, with the key difference being the presence of organic groups instead of inorganic ions. It also touches on the concept of making the free base from an amine salt, a common process in organic chemistry.
📉 Understanding Acidity: The PKA Scale
This paragraph delves into the PKA scale, which measures the degree of acidity through the negative logarithm of the acid dissociation constant (Ka). The summary explains how PKA values can indicate the strength of an acid, with lower PKA values corresponding to stronger acids. It provides examples ranging from strong acids like HCl to very weak acids like methane, illustrating the vast difference in their dissociation in water.
🤔 The Significance of Electron Flow in Acid-Base Reactions
The speaker concludes by emphasizing the importance of understanding electron flow in acid-base reactions, particularly in the context of organic chemistry. The summary highlights the role of lone pair movement in these reactions and sets the stage for further discussions on acid strength and the factors influencing it.
Mindmap
Keywords
💡Molecular Geometry
💡sp3 Hybridization
💡VSEPR Theory
💡Electronegativity
💡Ammonia
💡Water
💡Ethylene
💡Carbocation
💡Acidity and Basicity
💡Curved Arrow Notation
💡pKa
Highlights
Introduction to molecular geometry in organic chemistry, focusing on sp3 hybridized atoms and tetrahedral arrangements.
Explanation of bond angles in sp3 hybridized molecules like methane, with all bond angles being approximately 109.5°.
Transition from carbon to nitrogen, discussing the molecular geometry shift from tetrahedral to pyramidal in ammonia (NH3).
Molecular geometry of water (H2O), illustrating the bent structure due to the lone pairs of electrons on the oxygen atom.
VSEPR Theory review and its application to organic chemistry, including the impact of lone pairs on bond angles.
Introduction to sp2 hybridized atoms, with bond angles of approximately 120° and a trigonal planar arrangement.
Methyl carbocation (CH3+) structure and its planar trigonal geometry due to sp2 hybridization.
Discussion on s hybridized atoms, with bond angles of 180° and linear arrangements, exemplified by acetylene.
Explanation of the central role of acids and bases in organic chemistry, including their relationship to reactivity.
Introduction to curved arrow notation as a central concept in understanding organic reactions.
Differentiation between Bronsted-Lowry acids and bases and their role in proton transfer reactions.
Mechanism of acid-base reactions using curved arrow notation to illustrate electron flow.
Comparison of acid-base reactions in inorganic and organic compounds, such as the reaction between methanol and HCl.
Discussion on the concept of 'free base' in the context of organic chemistry and its practical applications.
Introduction to PKA as a measure of acidity, explaining its significance in characterizing the strength of acids.
Explanation of the logarithmic scale of PKA and its implications for understanding differences in acid strength.
Overview of the range of PKA values from very strong acids like HCl to very weak acids like methane.
Transcripts
Browse More Related Video

Acids and Bases - Basic Introduction - Organic Chemistry

What Is The Bronsted Lowry Theory | Acids, Bases & Alkali's | Chemistry | FuseSchool
Acids and Bases, pH and pOH

3.1 Introduction to Acids and Bases | Organic Chemistry

Acidity: Crash Course Organic Chemistry #11

Introduction to Acid-Base Chemistry - AP Chemistry Unit 4, Topic 8
5.0 / 5 (0 votes)
Thanks for rating: