Acidity: Crash Course Organic Chemistry #11
TLDRThis Crash Course Organic Chemistry episode, hosted by Deboki Chakravarti, delves into acid-base chemistry, a fundamental concept for predicting chemical reactions. The video explains the Brønsted-Lowry definition of acids and bases, where an acid is a proton donor and a base is a proton acceptor. It introduces the acid dissociation constant (Ka) and its negative logarithm (pKa) to compare the strength of acids. The episode explores factors affecting acidity, including resonance stabilization, atom identity, the inductive effect, and the s character of orbitals. Using examples like acetic acid, phenol, and thiophenol, the video illustrates how these factors influence a molecule's tendency to lose or gain a proton. The content is engaging, providing a solid foundation for understanding the role of acids and bases in organic chemistry.
Takeaways
- 🎬 The sci-fi movie 'Alien' features a xenomorph with acidic blood, serving as an engaging introduction to the topic of acid strength.
- 🧪 Organic chemists use the concept of acids and bases to predict chemical reactions, which is crucial for understanding molecular interactions.
- 📚 The Brønsted-Lowry definition of acids and bases, proposed in 1923, is the focus of this lesson, where an acid is a proton donor and a base is a proton acceptor.
- ⚛️ Electron pushing is a technique used to visualize how bonds break and form, which is fundamental to understanding acid-base reactions.
- 💧 Water acts as a base by removing a proton from carboxylic acids like acetic and propanoic acid, forming a hydronium ion and a carboxylate ion.
- 📉 The acid dissociation constant (Ka) is a measure of a molecule's willingness to lose a proton and is used to determine if a molecule is a strong or weak acid.
- 🔢 pKa, the negative log of Ka, is used to compare the strength of different acids, with a lower pKa indicating a stronger acid and a higher pKa indicating a weaker acid.
- 🔁 Resonance stabilization plays a significant role in the acidity of molecules, as seen in the comparison between acetic acid and ethanol, influencing how easily a molecule can lose a proton.
- 📐 The size and electronegativity of atoms, as well as their polarizability, affect the stability of conjugate bases and thus the pKa values of acids.
- 🔗 The inductive effect, related to electronegativity differences within a molecule, can stabilize a negative charge and thus influence a molecule's acidity.
- 🧠 Understanding the s character of hybrid orbitals is essential for predicting the acidity of molecules, as sp hybridized orbitals can stabilize negative charges more effectively than sp2 or sp3.
Q & A
What is the significance of the xenomorph's acidic blood in the movie 'Alien'?
-The xenomorph's acidic blood is significant as it is portrayed as being stronger than most acids produced by Earth creatures, which adds to the creature's threat level and creates a memorable and creepy detail in the sci-fi movie.
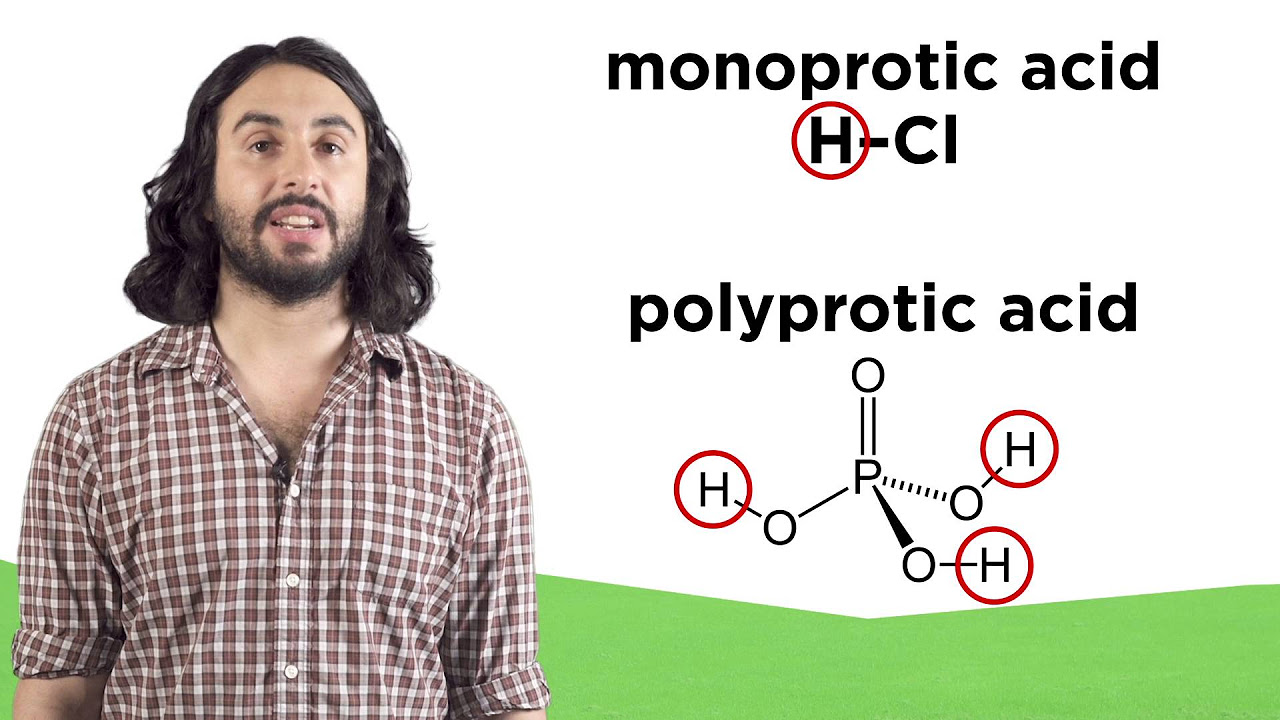
What is the Brønsted-Lowry definition of an acid?
-In the Brønsted-Lowry definition, an acid is anything that loses a proton (a plus-one-charged hydrogen ion).
How is a base defined in the Brønsted-Lowry theory?
-A base, according to the Brønsted-Lowry theory, is anything that accepts a proton.
What is the role of water when carboxylic acids like acetic acid and propanoic acid are dissolved in it?
-When carboxylic acids are dissolved in water, water acts as a base and removes a proton to form a hydronium ion and the corresponding carboxylate ion.
What is the Ka value in the context of acid-base chemistry?
-Ka, or the acid dissociation constant, describes the relationships between products and reactants when the rate of the forward reaction is equal to the reverse reaction, indicating that the reaction has reached equilibrium.
How can the negative log of Ka, known as pKa, be used to compare the strength of two acids?
-A lower pKa indicates that the equilibrium favors the product side, meaning the molecule is a stronger acid. Conversely, a higher pKa means the equilibrium favors the reactant side, indicating a weaker acid.
Why is acetic acid considered a stronger acid than ethanol?
-Acetic acid is a stronger acid than ethanol because when it loses a proton, it forms its conjugate base, acetate, which has resonance stabilization that allows the negative charge to be distributed over two oxygen atoms, making it easier for acetic acid to lose a proton.
How does the presence of resonance structures affect the acidity of a compound?
-The presence of resonance structures in a compound's conjugate base allows for the distribution of the negative charge across multiple atoms, which increases the stability of the conjugate base and makes the corresponding acid more acidic.
What is the inductive effect and how does it influence the pKa of a compound?
-The inductive effect is related to electronegativity throughout a molecule, where more electronegative atoms pull negative charges toward them through bonds. This effect can stabilize the conjugate base, influencing the pKa by favoring the products side of the equilibrium in more electronegative compounds.
How does the s character of hybrid orbitals impact the acidity of a molecule?
-The s character of hybrid orbitals affects acidity because orbitals with more s character, like sp hybrid orbitals, hold electrons closer to the nucleus, which allows the atom to stabilize a negative charge better, making the corresponding acid more acidic.
What are the four major factors that help us understand the role of pKa in reactions?
-The four major factors are: 1) Atom identity - More electronegative and larger elements stabilize charge better, 2) Resonance stabilization - Multiple Lewis structures for conjugate bases increase stability, 3) The inductive effect - Electronegative atoms stabilize conjugate bases through covalent bonds, and 4) The s character of the orbital - More s character results in better stabilization of negative charge.
Why is thiophenol more acidic than phenol, despite their structural similarity?
-Thiophenol is more acidic than phenol because the sulfur atom in thiophenol is larger and more polarizable than the oxygen atom in phenol. This means that in thiophenol's conjugate base, the electrons are more smeared out, stabilizing the conjugate base and making thiophenol more acidic.
Outlines
🌟 Introduction to Acid-Base Chemistry
The first paragraph introduces the topic of organic chemistry, focusing on acid-base chemistry. Deboki Chakravarti begins by referencing the sci-fi movie 'Alien' to illustrate the concept of strong acids, comparing the xenomorph's acidic blood to the weak formic acid produced by Tawny ants. The paragraph then delves into the importance of understanding acid-base chemistry for predicting chemical reactions. The Brønsted-Lowry definition of acids and bases is introduced, where an acid is a substance that donates a proton and a base is one that accepts it. The concept of conjugate acids and bases is explained using the example of carboxylic acids in water. The Ka, or acid dissociation constant, is introduced as a measure of a molecule's tendency to donate a proton. The pKa, which is the negative log of Ka, is explained as a way to compare the strength of different acids. The paragraph concludes with examples of how the pKa values of hydrochloric acid, propanoic acid, acetic acid, and ethanol reflect their relative acid strengths and the role of resonance stabilization in this context.
🔬 Factors Influencing Acidity and pKa Values
The second paragraph explores the factors that influence the acidity of compounds and their pKa values. It begins by comparing ethanol and acetic acid, highlighting how the stability of the conjugate base affects the strength of the acid. The concept of resonance stabilization is further illustrated by comparing phenol and cyclohexanol, showing how the ability to distribute negative charge through resonance structures affects acidity. The paragraph then discusses how the identity of the atom that loses the proton, the electronegativity and size of atoms, and the polarizability of larger atoms contribute to the stability of conjugate bases and thus to acidity. Examples are given, such as the comparison between phenol and thiophenol, to show how the size and electronegativity of atoms affect pKa values. The inductive effect of electronegative atoms is explained using acetic acid and trifluoroacetic acid as examples. Lastly, the paragraph touches on the role of hybrid orbitals and their s character in stabilizing negative charges, using ethane, ethene, and ethyne to illustrate the concept.
🔑 Key Factors in Predicting Relative Acidity
The third and final paragraph summarizes the key factors that can be used to predict the relative acidity of compounds. It reiterates the four major factors that stabilize negative charge in a conjugate base: atom identity, resonance stabilization, the inductive effect, and the s character of the orbital. The paragraph emphasizes the importance of understanding these factors for predicting the outcomes of chemical reactions. It concludes with a teaser for the next episode, which will focus on forming covalent bonds at molecular hotspots. The viewer is also encouraged to support Crash Course on Patreon to keep the educational content free for everyone.
Mindmap
Keywords
💡Acidic Blood
💡Xenomorph
💡Formic Acid
💡Brønsted-Lowry Definition
💡Acid Dissociation Constant (Ka)
💡pKa
💡Resonance Stabilization
💡Electronegativity
💡Polarizability
💡Inductive Effect
💡Hybrid Orbitals
Highlights
Xenomorph blood from the movie 'Alien' is stronger than most Earth creatures' acids, which is used to illustrate the concept of acidity.
Tawny ants use formic acid as a defense mechanism, which is relatively weak compared to xenomorph blood.
Organic chemists predict the strength of weak acids and bases, which is crucial for understanding molecular reactions.
The Brønsted-Lowry definition of acids and bases is used for this discussion, focusing on the transfer of protons.
Acid-base chemistry is integral to understanding where reactions can occur within molecules.
The acid dissociation constant (Ka) describes the relationship between products and reactants at equilibrium.
A large Ka indicates a strong acid, while a small Ka indicates a weak acid.
The negative log of Ka (pKa) is used to compare the strength of different acids more conveniently.
Hydrochloric acid is a strong acid with a negative pKa, indicating complete dissociation.
The pKa values of propanoic acid and acetic acid are similar, indicating they are both weak acids.
Ethanol is a weaker acid than acetic acid due to differences in charge distribution and resonance stabilization.
Resonance stabilization, such as in phenol, affects the acidity of compounds by spreading the negative charge.
Atom size and polarizability influence acidity, with larger atoms stabilizing negative charges more effectively.
The inductive effect, related to electronegativity, also plays a role in stabilizing negative charges in conjugate bases.
The s character of hybrid orbitals affects acidity, with more s character resulting in better stabilization of negative charges.
Four key factors that predict relative acidity are identified: atom identity, resonance stabilization, the inductive effect, and the s character of the orbital.
Understanding acidity is vital for predicting the outcomes of chemical reactions in organic chemistry.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: