Chemistry | Organic Chemistry | Reactions (Substitution, Addition and Elimination)
TLDRThis educational video script delves into organic chemistry, focusing on the reactions of organic compounds. It explains combustion, substitution, addition, and elimination reactions, highlighting the differences in outcomes based on reaction conditions. Specific examples include the formation of alcohols from halogens and the application of Markovnikov's and Zaitsev's rules in predicting major products. The script also emphasizes the importance of balancing chemical equations and the exothermic nature of combustion reactions, aiming to equip viewers with a solid understanding of organic reactions and their applications.
Takeaways
- 🔍 The video script discusses various types of organic reactions, including combustion, substitution, addition, and elimination reactions.
- 🔥 In the context of combustion, alkanes react with oxygen to produce carbon dioxide and water, with complete combustion requiring an excess of oxygen.
- ⚖️ The script emphasizes the importance of balancing chemical equations, as demonstrated with the combustion of ethane (C2H6) to form carbon dioxide and water.
- 🌐 The concept of stoichiometry is highlighted, with the script explaining how to adjust coefficients to avoid fractions or decimals in balanced equations.
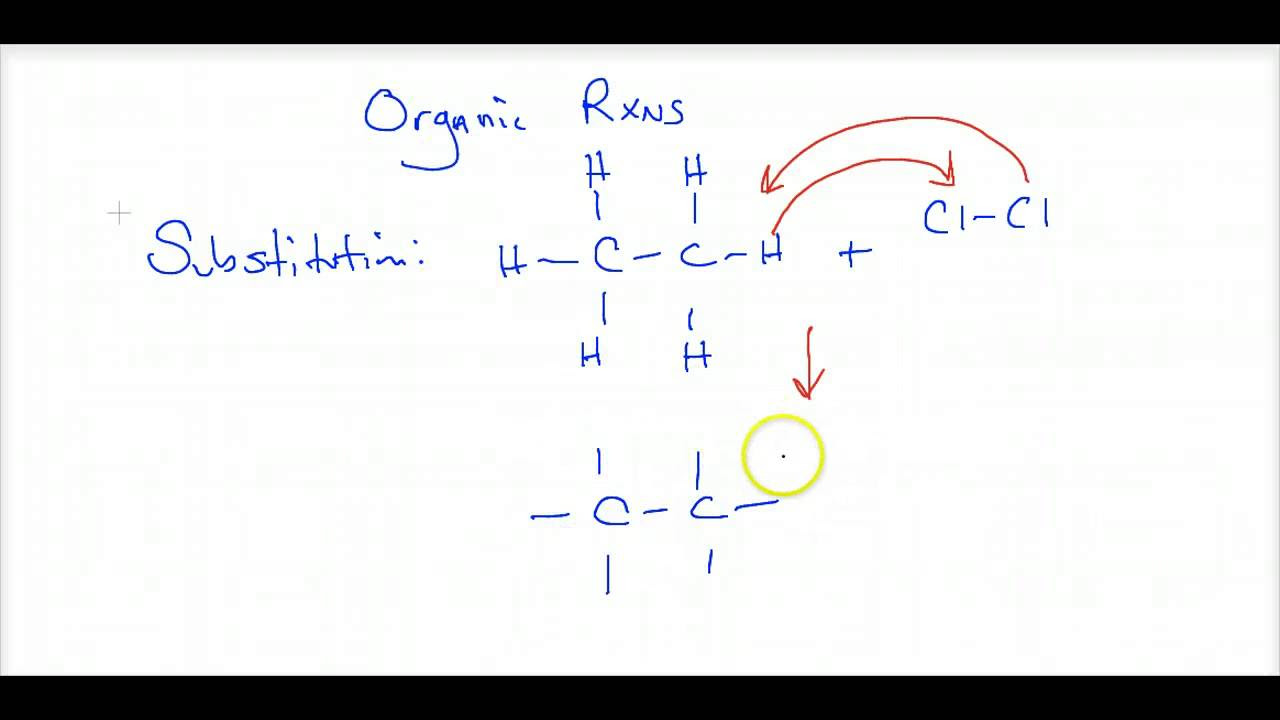
- 🔬 The video introduces the idea of substitution reactions, specifically halogenation, where hydrogen atoms in alkanes are replaced by halogen atoms under UV energy.
- 💧 Addition reactions are explained, showing how unsaturated hydrocarbons like alkenes can add molecules like hydrogen (hydrogenation) or halogens (halogenation) across a double bond.
- 📚 Markovnikov's rule is introduced, which predicts the major product of addition reactions to alkenes, stating that the hydrogen atom of water or hydrogen halide will add to the carbon with more hydrogens.
- 🌱 The script touches on elimination reactions, where molecules like alkanes can lose atoms or groups to form alkenes, sometimes referred to as 'cracking' in the context of oil refining.
- ⚛️ Zaitsev's rule is presented as a principle for predicting the major product of elimination reactions, favoring the formation of a more substituted alkene.
- 🧪 The difference between reactions under different conditions is illustrated, such as the formation of an alcohol versus an alkene depending on whether a dilute or concentrated base is used.
- 📝 The video concludes with a mention of future lessons on answering past exam questions, indicating the practical application of understanding these organic reactions.
Q & A
What is the primary focus of the video script?
-The primary focus of the video script is to explain various types of organic reactions, including combustion, substitution, addition, and elimination reactions, and to provide examples of how these reactions occur with different organic compounds.
What is the difference between complete and incomplete combustion of an alkane?
-Complete combustion of an alkane occurs in the presence of excess oxygen and results in the formation of carbon dioxide and water. Incomplete combustion happens when there is not enough oxygen, leading to the production of carbon monoxide instead of carbon dioxide.
How is the combustion reaction of an alkane balanced?
-The combustion reaction of an alkane is balanced by adjusting the coefficients in front of the reactants and products to ensure that the number of atoms for each element is the same on both sides of the equation. This involves multiplying the coefficients by the lowest common denominator to avoid fractions or decimals.
What is a substitution reaction in the context of organic chemistry?
-A substitution reaction in organic chemistry is a type of reaction where an atom or a group of atoms in a molecule is replaced by another atom or group of atoms. For example, when an alkane reacts with a halogen in the presence of UV energy, a hydrogen atom is substituted by a halogen atom.
What is an addition reaction and how does it differ from a substitution reaction?
-An addition reaction is a type of chemical reaction where atoms or groups of atoms are added to a molecule, often breaking an unsaturated bond and forming new bonds with the added atoms. This differs from a substitution reaction, where one atom or group is replaced by another rather than being added.
Can you explain what Markovnikov's rule states and its significance in organic chemistry?
-Markovnikov's rule states that in the addition of a proton (H+) and a hydroxide ion (OH-) to an unsymmetrical alkene, the hydrogen atom will tend to add to the carbon with fewer hydrogen atoms, and the hydroxide ion will add to the carbon with more hydrogen atoms. This rule helps predict the major product in such addition reactions.
What is an elimination reaction and what are its characteristics?
-An elimination reaction is a type of organic reaction where one or more atoms or groups of atoms are removed from a molecule, often resulting in the formation of a double bond. This reaction can lead to the formation of smaller molecules or the creation of unsaturated compounds from saturated ones.
What is Zaitsev's rule and how does it apply to elimination reactions?
-Zaitsev's rule, also known as the rule of least substitution, states that in an elimination reaction, the major product will be the one formed by the removal of a hydrogen atom from the carbon with the fewest hydrogen atoms. This rule helps predict the major product when multiple elimination pathways are possible.
What is the significance of the type of base used in reactions involving halogens in organic chemistry?
-The type of base used can determine the type of reaction that occurs. For instance, a dilute strong base like sodium hydroxide can lead to a substitution reaction, forming an alcohol, while concentrated sodium hydroxide at high temperatures can lead to an elimination reaction, forming an alkene and a halogen salt.
What is meant by the term 'dehydrohalogenation' in the context of elimination reactions?
-Dehydrohalogenation refers to an elimination reaction where a hydrogen atom and a halogen atom are removed from a molecule, resulting in the formation of a double bond. This term specifically indicates the removal of both hydrogen and halogen atoms.
How are the concepts of major and minor products relevant in organic reactions?
-The concepts of major and minor products are relevant as they indicate the predominance of one product over another in a chemical reaction. The major product is the most abundant, formed according to specific rules like Markovnikov's or Zaitsev's, while the minor product is formed in lesser quantities through alternative pathways.
Outlines
📚 Introduction to Organic Reactions
The video script begins with an introduction to organic reactions, following a comprehensive coverage of organic compound naming. The instructor invites viewers to subscribe and reach out with questions or to discuss past papers. The first topic of discussion is the combustion reaction, which involves hydrocarbons reacting with oxygen to produce carbon dioxide and water, with complete combustion yielding these products in the presence of excess oxygen, and incomplete combustion resulting in carbon monoxide. An example of balancing the combustion reaction of ethane (C2H6) is provided to demonstrate the process of balancing chemical equations.
🔍 Balancing Combustion Reactions
This paragraph delves deeper into the process of balancing combustion reactions, using the example of propane (C3H8) to illustrate the steps. The instructor explains how to account for the correct number of atoms on both sides of the equation by adjusting coefficients, resulting in a balanced chemical equation. The concept of stoichiometric ratios is introduced, emphasizing the preference for whole numbers over fractions or decimals in chemical equations.
🔥 Exothermic Nature of Combustion Reactions
The script highlights that combustion reactions are exothermic, meaning they release energy. The instructor uses the example of petrol (C8H18) combustion, a reaction that occurs in car engines, to explain this concept. The release of energy is represented by a negative change in enthalpy (ΔH < 0), a fundamental principle in thermochemistry.
🌐 Reactions of Saturated and Unsaturated Hydrocarbons
The instructor contrasts the reactions of saturated and unsaturated hydrocarbons. Saturated hydrocarbons, such as alkanes, typically undergo substitution reactions with halogens, facilitated by UV energy, leading to the formation of haloalkanes and hydrogen halides. Unsaturated hydrocarbons, including alkenes and alkynes, are introduced, with their distinct reactions to be discussed in subsequent paragraphs.
🔄 Substitution and Addition Reactions in Hydrocarbons
This section explains the difference between substitution and addition reactions in hydrocarbons. Alkanes, with only single bonds, mainly undergo substitution reactions where hydrogen atoms are replaced by halogen atoms. In contrast, alkenes, containing double bonds, primarily undergo addition reactions where the double bond breaks and the components of the reactant add across the former double bond, leading to the formation of new products.
🌀 Markovnikov's Rule in Addition Reactions
The instructor introduces Markovnikov's rule, which predicts the major product in the addition of a proton (H+) and a nucleophile (such as OH-) to an alkene. According to the rule, the hydrogen atom will preferentially add to the carbon with the greater number of hydrogen atoms, resulting in the formation of the major product. This principle is illustrated with the example of an alkene reacting with water (hydration) and hydrogen chloride (hydrohalogenation).
⚡ Elimination Reactions in Organic Chemistry
The script moves on to discuss elimination reactions, where a molecule loses one or more small molecules (like H2O or HCl), forming an unsaturated compound such as an alkene. The process of cracking in petroleum refining is mentioned as a real-world application of elimination reactions, where large hydrocarbon molecules are broken down into smaller, more useful ones like gasoline and jet fuel.
📉 Zaitsev's Rule in Elimination Reactions
Zaitsev's rule is introduced, which states that in an elimination reaction, the hydrogen atom will be preferentially removed from the carbon with the fewest hydrogen atoms, leading to the formation of the more stable alkene. This rule is applied to predict the major product of an elimination reaction involving an alkane with a halogen substituent.
🔄 Hydrolysis and Dehydrohalogenation Reactions
The final paragraph covers hydrolysis and dehydrohalogenation reactions. Hydrolysis involves the reaction of a compound with water, often catalyzed by an acid or base. Dehydrohalogenation is a specific type of elimination reaction where a hydrogen and a halogen are removed from a molecule, forming a double bond and a hydrogen halide. The conditions that favor each reaction type are discussed, highlighting the importance of understanding reaction mechanisms in organic chemistry.
Mindmap
Keywords
💡Combustion
💡Alkane
💡Halogenation
💡Addition Reaction
💡Substitution Reaction
💡Makarov's Rule
💡Hydrogenation
💡Elimination Reaction
💡Dehydrogenation
💡Zaitsev's Rule
💡Hydrolysis
Highlights
Introduction to organic reactions and the importance of understanding how organic compounds interact.
Explanation of complete and incomplete combustion reactions involving alkanes and oxygen.
Demonstration of balancing chemical equations for combustion reactions.
Discussion on the types of hydrocarbons: alkanes, alkenes, and alkynes, and their reactions.
Description of substitution reactions in alkanes, including halogenation and the role of UV energy.
Illustration of addition reactions in alkenes, such as halogenation and hydrogenation.
Introduction of Markovnikov's rule in determining the major product of addition reactions.
Explanation of the difference between dilute and concentrated bases in organic reactions.
Hydrolysis reaction as an example of an elimination reaction leading to the formation of an alkene.
Zaitsev's rule for predicting the major product in elimination reactions.
Differentiation between dehydrogenation and dehydrohalogenation in elimination reactions.
The concept of cracking in the context of breaking down large organic molecules into smaller, more usable ones.
Combination of hydrogen and halogen in addition reactions leading to products like 1,2-dichloroethane.
The role of molar ratios and stoichiometry in balancing chemical equations.
Practical application of organic reactions in the formation of petrol and other fuels.
Importance of understanding reaction mechanisms for answering past exam questions.
Invitation for students to reach out with questions or for assistance with past papers.
Transcripts
Browse More Related Video

Chemical Properties of Carbon Compounds
Crash Course Regents Chemistry 12 - Reaction Review

Mechanisms | Explained | Year 12 or AS Chemistry | Organic Chemistry | A level Chemistry

Organic Chemistry 1 Final Exam Review

7.1 SN2 Reaction | Organic Chemistry

Lec-08 I Types of organic reactions I Applied Chemistry I Chemical engineering
5.0 / 5 (0 votes)
Thanks for rating: