Crash Course Regents Chemistry 12 - Reaction Review
TLDRThis video script from a Regents Chemistry series covers various chemical reactions relevant to the New York State curriculum. It discusses synthesis, analysis, decomposition, combustion, ionization, double replacement, single replacement, and organic reactions like substitution, addition, esterification, fermentation, polymerization, and nuclear reactions. The script provides insights into reaction types, balancing equations, and understanding redox reactions.
Takeaways
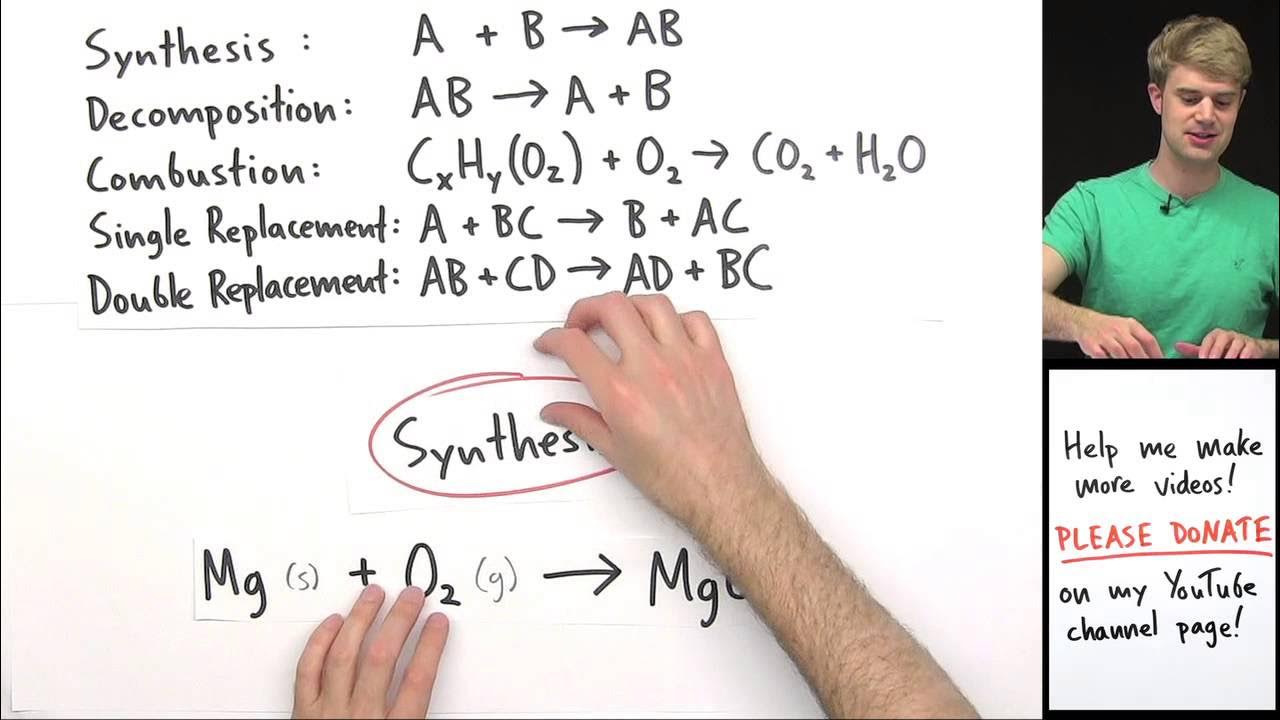
- 🔬 Synthesis reactions involve combining elements to form compounds, often from their elemental states, and are also known as formation or redox reactions due to changes in oxidation states.
- 🔍 Analysis or decomposition reactions are the reverse of synthesis, breaking compounds down into their elemental states, and can also be referred to as ionization reactions when involving salts dissolving in water.
- ⚡ Combustion reactions typically involve organic fuels reacting with oxygen to produce CO2 and water, releasing energy and are often exothermic.
- 🌊 Ionization reactions describe the process of ionic compounds breaking apart into their constituent ions when dissolved in water, which can be either endothermic or exothermic.
- 💥 Double replacement reactions occur between aqueous salts, exchanging ions to form new compounds, potentially resulting in precipitates if the products are insoluble.
- 🔄 Single replacement reactions involve one element replacing another in a compound, often resulting in a redox reaction where one element is oxidized and another is reduced.
- 🌱 Organic chemistry includes substitution reactions where hydrogen atoms in saturated hydrocarbons are replaced by other atoms or groups, such as chlorine in the case of chlorination.
- 🌀 Addition reactions in unsaturated hydrocarbons involve adding atoms across a double bond, resulting in only one product, unlike substitution which yields two products.
- 🍶 Esterification is a dehydration reaction where an organic acid and an alcohol react to form an ester and water, following the general formula R-CO-O-R' + H2O.
- 🍺 Fermentation is an example of esterification where sugars are converted into ethanol and CO2 by the action of yeast and enzymes in an anaerobic glycolysis process.
- 🧪 Polymerization involves linking monomers into long-chain polymers, either through addition polymerization where double bonds are opened to add monomers, or condensation polymerization where water or other small molecules are removed.
Q & A
What is a synthesis reaction in chemistry?
-A synthesis reaction is a type of chemical reaction where two or more elements or simpler compounds combine to form a more complex compound. It is often represented by the formation of a compound from its elemental states.
What is another term for a synthesis reaction?
-A synthesis reaction can also be referred to as a formation reaction, indicating the formation of a new compound from its constituent elements or simpler compounds.
Why are synthesis reactions also considered redox reactions?
-Synthesis reactions are considered redox reactions because they involve a change in oxidation states of the elements involved. For example, in the formation of NO2 from nitrogen and oxygen, the oxidation states of nitrogen and oxygen change, indicating a transfer of electrons.
What is the difference between synthesis and analysis reactions?
-Synthesis reactions involve the combination of elements or simpler compounds to form a more complex compound, while analysis reactions involve the breakdown of a compound into its elemental states or simpler compounds to understand its composition.
What are the characteristics of a combustion reaction?
-A combustion reaction typically involves an organic fuel, such as propane, reacting with oxygen to produce carbon dioxide, water, and energy in the form of heat and light. These reactions are exothermic, releasing energy.
How can you predict if a precipitate will form in a double replacement reaction?
-To predict if a precipitate will form in a double replacement reaction, you can refer to solubility guidelines or 'Table F' which provides information on the solubility of different ionic compounds in water. If the reaction results in an insoluble compound, a precipitate will form.
What is a single replacement reaction and how does it differ from a double replacement reaction?
-A single replacement reaction involves one element replacing another in a compound. It differs from a double replacement reaction, where two elements switch places between two different compounds. Single replacement reactions often involve a standalone element and are indicative of redox processes.
What is esterification and how is it related to the formation of esters?
-Esterification is a chemical reaction between an organic acid and an alcohol that results in the formation of an ester and water. It is the process by which esters are formed, involving the removal of a water molecule and the creation of an ester linkage.
What is the significance of the term 'hydrolysis' in organic chemistry?
-Hydrolysis in organic chemistry refers to the reaction where a molecule is cleaved into two parts by the addition of a water molecule. This process is commonly involved in the breakdown of esters and other compounds in the presence of water.
What is polymerization and how does addition polymerization differ from condensation polymerization?
-Polymerization is the process of forming polymers from monomers. Addition polymerization involves the opening of double bonds in monomers and linking them together, while condensation polymerization involves the linking of monomers with the concurrent release of small molecules, such as water.
What is the fundamental principle behind nuclear fission and fusion reactions?
-The fundamental principle behind nuclear fission is the splitting of a large atomic nucleus into smaller fragments, often releasing neutrons and energy. In contrast, nuclear fusion involves the combining of light nuclei to form a heavier nucleus, which also releases energy and may involve the formation of helium from hydrogen nuclei.
Outlines
🔬 Chemistry Reactions Overview
This paragraph introduces the various types of chemical reactions covered in the New York State Regents chemistry curriculum. It starts with synthesis reactions, where elements combine to form compounds, exemplified by nitrogen and oxygen forming NO2. The paragraph also touches on analysis reactions, which are the reverse of synthesis, breaking down compounds into elements. The speaker discusses redox reactions, where oxidation states change, and uses the example of NO2 formation to illustrate this. The paragraph further explains that synthesis reactions are exothermic, releasing energy, while decomposition reactions are endothermic, requiring energy input. The speaker also mentions that these reactions can be found in Table I of the curriculum, highlighting the importance of understanding the formation and decomposition of compounds.
🌊 Ionization and Double Replacement Reactions
This paragraph delves into ionization reactions, where salts dissolve in water, breaking into their constituent ions. The speaker explains that this can be exothermic or endothermic, depending on the salt. The paragraph then moves on to double replacement reactions, where aqueous salts exchange ions to form new compounds. The example given is the reaction between lead nitrate and potassium iodide, resulting in the formation of a precipitate. The speaker emphasizes the importance of balancing chemical equations and using Table F (solubility guidelines) to predict whether a precipitate will form. The paragraph concludes with a discussion on single replacement reactions, where one element replaces another in a compound, illustrated by the reaction of copper with silver nitrate to form copper nitrate and solid silver.
🔋 Redox and Organic Reactions
The speaker begins by discussing redox reactions, focusing on single replacement reactions and their spontaneity. The example of copper and silver is used to explain oxidation and reduction processes. The paragraph then transitions to organic chemistry, starting with substitution reactions in saturated hydrocarbons, where hydrogen atoms are replaced by other atoms, such as chlorine. The speaker contrasts this with addition reactions in unsaturated hydrocarbons, where double bonds allow for the addition of atoms across the bond. The paragraph concludes with a brief mention of esterification, a type of organic reaction where an acid and an alcohol react to form an ester and water.
🍻 Fermentation and Polymerization
This paragraph explores fermentation, a process where sugars are converted into ethanol and carbon dioxide by yeast through anaerobic glycolysis. The speaker explains that this process is used in the production of alcoholic beverages like beer and wine. The paragraph then discusses polymerization, starting with addition polymerization, where monomers with double bonds are joined to form long-chain polymers. The speaker contrasts this with condensation polymerization, where monomers are joined by releasing a small molecule, such as water, to form polymers like polyesters. The paragraph concludes with a brief mention of saponification, a process where fats and oils are reacted with a strong base to form soap and glycerol.
⚛️ Nuclear Fission and Fusion
The final paragraph covers nuclear reactions, starting with fission, where a large atomic nucleus splits into smaller fragments, releasing energy and neutrons. The speaker uses the example of uranium splitting into barium and krypton, along with the release of neutrons. The paragraph then discusses fusion, where light nuclei combine to form heavier nuclei, such as helium. The speaker explains that fusion requires a significant amount of energy to initiate but releases more energy than fission. The paragraph concludes with a reference to Einstein's mass-energy equivalence formula, E = mc^2, highlighting the mass defect in nuclear reactions and the energy released as a result.
Mindmap
Keywords
💡Synthesis Reaction
💡Analysis Reaction
💡Redox Reaction
💡Combustion Reaction
💡Decomposition Reaction
💡Ionization Reaction
💡Double Replacement Reaction
💡Single Replacement Reaction
💡Substitution Reaction
💡Addition Reaction
💡Esterification
💡Fermentation
💡Polymerization
💡Saponification
💡Nuclear Fission
💡Nuclear Fusion
Highlights
Introduction to the review of chemical reactions and their names as per the New York State Regents chemistry curriculum.
Explanation of synthesis reactions, including the formation of NO2 from nitrogen and oxygen.
Clarification that synthesis reactions can also be called formation or redox reactions due to changes in oxidation states.
Introduction to analysis reactions as the opposite of synthesis, breaking down compounds to elemental states.
The role of Table I in identifying synthesis and decomposition reactions among elements.
The relationship between exothermic and endothermic reactions and their ΔH (enthalpy change) values.
Combustion reactions involving organic fuels and oxygen, producing CO2 and water.
Identification of ionization reactions as processes where salts dissolve in water and dissociate into ions.
Double replacement reactions involving aqueous salts and the formation of precipitates.
Use of Table F for predicting the solubility of compounds and the formation of precipitates.
Single replacement reactions where one element replaces another in a compound, often involving redox processes.
The spontaneity of reactions and the role of reference tables in predicting redox outcomes.
Organic chemistry reactions, starting with substitution reactions in saturated hydrocarbons.
Addition reactions in unsaturated hydrocarbons where double bonds are involved.
Esterification, the process of making esters from organic acids and alcohols with the release of water.
Fermentation as an example of esterification, producing ethanol and CO2 from sugars.
Polymerization, the process of linking monomers to form long-chain polymers.
Condensation polymerization, where water is released as esters are formed.
Saponification, the reaction of esters with a strong base to form soap and glycerol.
Nuclear fission and fusion reactions, explaining the difference between splitting and combining atomic nuclei.
The concept of mass defect in nuclear reactions and its relation to energy release (E=mc²).
Transcripts
Browse More Related Video

Types of Chemical Reactions

4.2 Types of Chemical Reactions | High School Chemistry

Chemical Reactions

AP Chemistry Unit 4 Review: Chemical Reactions
Classifying Types of Chemical Reactions Practice Problems

Chemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry
5.0 / 5 (0 votes)
Thanks for rating: