Mechanisms | Explained | Year 12 or AS Chemistry | Organic Chemistry | A level Chemistry
TLDRThis A-Level chemistry video offers an in-depth exploration of organic mechanisms, focusing on electrophilic addition, nucleophilic substitution, and elimination reactions. It explains the importance of understanding mechanisms for tracking electron movement during chemical reactions, using curly arrows to depict this process. The video delves into the specifics of each reaction type, illustrating how electrophiles are attracted to electron-rich alkenes, the role of nucleophiles in substitution, and the conditions influencing elimination reactions. It also discusses the formation of major and minor products, the stability of carbocations, and the implications for students when approaching exam questions.
Takeaways
- 📚 The video script provides an overview of organic mechanisms covered in year one chemistry, focusing on electrophilic addition, nucleophilic substitution, and elimination.
- 🧬 Mechanisms are used to illustrate the movement of electrons during chemical reactions, showing bond formation and breaking, which is essential for understanding reaction processes.
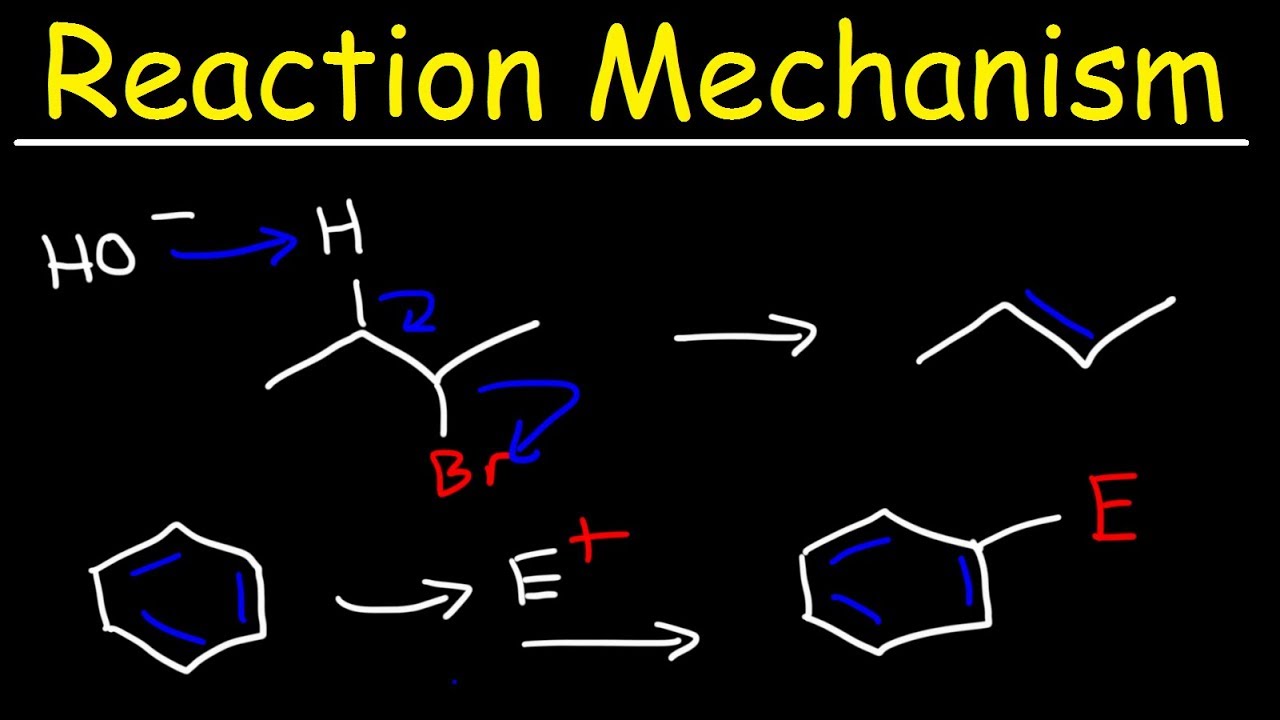
- 👉 Curly arrows in mechanism diagrams start at a lone pair or a bond about to break and point to a new bond or atom gaining a lone pair, representing electron movement.
- 🌐 Electrophilic addition occurs with alkenes reacting with electrophiles like hydrogen halides, bromine, or sulfuric acid, resulting in the formation of halogenoalkanes.
- 🔍 The stability of carbocations plays a crucial role in determining the major and minor products of electrophilic addition reactions, with more stable carbocations leading to the major products.
- 💧 Nucleophilic substitution involves nucleophiles replacing a halogen in a halogenoalkane, with the mechanism differing based on the nucleophile used, such as hydroxide, cyanide, or ammonia.
- 🔄 Carbocation stability decreases from tertiary to secondary to primary, impacting the outcome of reactions and the formation of major and minor products.
- ⚗️ Energy profile diagrams can be used to justify the stability of carbocations, where more stable carbocations have lower activation energies and are formed more readily.
- 🚫 Ammonia as a nucleophile leads to the formation of amines, with the reaction requiring two steps and involving the removal of a proton by another ammonia molecule to avoid multiple substitutions.
- 🌡️ The conditions of the reaction, such as temperature and solvent, can influence whether elimination or substitution occurs, with higher temperatures favoring elimination.
- 🔄 Elimination reactions, such as dehydration of alcohols, can lead to the formation of alkenes and multiple isomers, highlighting the complexity of organic chemistry reactions.
Q & A
What are the main types of organic mechanisms discussed in the video?
-The main types of organic mechanisms discussed in the video are electrophilic addition, nucleophilic substitution, and elimination.
Why are mechanisms used in organic chemistry?
-Mechanisms are used in organic chemistry to show the movement of electrons during a chemical reaction, allowing us to see how a reaction proceeds by identifying the bonds that are breaking and those that are forming.
What are curly arrows used to represent in mechanism drawings?
-Curly arrows are used to represent the movement of electrons, specifically the flow of a pair of electrons, in mechanism drawings.
What is an electrophile and how does it relate to electrophilic addition?
-An electrophile is a species that seeks electrons and will join onto another molecule. In electrophilic addition, electrophiles are attracted to electron-rich areas, such as the double bond in alkenes, and participate in the addition reaction.
What are the common electrophiles that react with alkenes in electrophilic addition?
-The common electrophiles that react with alkenes in electrophilic addition include hydrogen halides like hydrogen bromide, bromine as a molecule or other halogens, and concentrated sulfuric acid.
How does the orientation of bromine (Br2) change when it approaches an alkene?
-When Br2 approaches an alkene, the electron-rich double bond repels the bonding electrons between the two bromine atoms slightly away from the double bond and towards one of the bromine atoms, inducing a dipole in the Br2 molecule.
What is a carbocation and why is it unstable?
-A carbocation is a carbon-based, positively charged intermediate species formed during electrophilic addition reactions. It is unstable because carbon atoms prefer to have four bonds, and in a carbocation, the positively charged carbon has more than four bonds temporarily.
What determines the major and minor products in the reaction of unsymmetrical alkenes with electrophiles?
-The major and minor products are determined by the stability of the carbocation intermediates formed during the reaction. The more stable the carbocation, the more likely it is to form the major product.
How does the stability of carbocations vary with the number of alkyl groups attached to the positively charged carbon?
-The stability of carbocations increases with the number of alkyl groups attached to the positively charged carbon. Tertiary carbocations are more stable than secondary, which are more stable than primary carbocations.
What is nucleophilic substitution and what are the common nucleophiles involved in this reaction?
-Nucleophilic substitution is a reaction where a nucleophile, a species with a lone pair of electrons, replaces another atom or group in a molecule. Common nucleophiles include the hydroxide ion, cyanide ion, and ammonia.
What are the two types of elimination reactions discussed in the video for A-Level chemistry?
-The two types of elimination reactions discussed are those involving halogenoalkanes and potassium hydroxide in ethanol, and those involving alcohols and concentrated acid or a heated catalyst like aluminum oxide.
How does the solvent affect the mechanism of reaction between halogenoalkanes and potassium hydroxide?
-When potassium hydroxide is dissolved in ethanol solvent, it favors elimination reactions, leading to the formation of alkenes. In contrast, when hydroxide ions are in water, they act as nucleophiles, favoring substitution reactions.
What is the role of the hydroxide ion in both elimination and nucleophilic substitution reactions?
-In elimination reactions, the hydroxide ion acts as a base, accepting a hydrogen ion from the adjacent carbon to the halogen. In nucleophilic substitution, the hydroxide ion acts as a nucleophile, attacking the electron-deficient carbon in the halogenoalkane.
Why does the reaction of alcohols with concentrated sulfuric acid or a heated catalyst lead to the formation of alkenes?
-The reaction involves the protonation of the alcohol oxygen, followed by the breaking of the carbon-oxygen bond and the adjacent carbon-hydrogen bond, leading to the formation of a double bond and the elimination of water, thus forming an alkene.
What determines the type of alkene formed in elimination reactions involving unsymmetrical halogenoalkanes?
-The type of alkene formed depends on which hydrogen is removed from the carbon adjacent to the halogen. Different hydrogen removal leads to the formation of different alkenes, which can be either primary or secondary products.
How can mixtures of organic products formed in reactions be separated?
-Mixtures of organic products can be separated using fractional distillation, which takes advantage of the different boiling points of the components in the mixture.
Outlines
🔍 Introduction to Organic Mechanisms
This paragraph introduces the topic of organic mechanisms, focusing on year one chemistry concepts such as electrophilic addition, nucleophilic substitution, and elimination. It emphasizes the importance of understanding mechanisms to identify reaction processes in exam questions. The explanation of how to represent electron movement using curly arrows is provided, with a distinction between single and double-headed arrows for different educational requirements. The paragraph also introduces the concept of electrophilic addition reactions involving alkenes and common electrophiles like hydrogen halides, bromine, and concentrated sulfuric acid.
🌐 Electrophilic Addition Mechanism
This paragraph delves into the specifics of electrophilic addition mechanisms, particularly with alkenes. It explains the process using ethene and hydrogen bromide as an example, detailing the formation of a carbocation intermediate and the subsequent steps leading to the final product, bromoethane. The paragraph also discusses the orientation of bromine molecules when reacting with alkenes and the resulting products, such as 1,2-dibromoethane. Additionally, it touches on the concept of major and minor products when dealing with unsymmetrical alkenes, explaining how carbocation stability influences the outcome of the reaction.
🔬 Carbocation Stability and Reaction Outcomes
Carbocation stability is the central theme of this paragraph, which explains how the stability of these intermediates affects the major and minor products of reactions. It outlines the stability hierarchy from tertiary to primary carbocations and uses energy profile diagrams to justify this order. The paragraph also discusses how the stability of carbocations influences the ease of their formation and the likelihood of them being the major product in a reaction.
🧬 Nucleophilic Substitution Mechanism
This section explores nucleophilic substitution mechanisms, where nucleophiles such as potassium hydroxide, sodium hydroxide, potassium cyanide, and ammonia replace halogens in halogenoalkanes. The paragraph explains the role of nucleophiles, which are electron-rich species attracted to electron-deficient atoms. It details the mechanism using iodoethane and the cyanide ion, illustrating the formation of a new bond and the departure of the iodine as an iodide ion. The unique behavior of ammonia as a nucleophile and its subsequent steps to form an amine and ammonium iodide is also discussed.
🌡 Conditions Influencing Reaction Mechanisms
The influence of reaction conditions on the type of mechanism that occurs is the focus of this paragraph. It contrasts the conditions required for nucleophilic substitution versus elimination reactions, explaining how temperature and solvent choice can lead to different outcomes. The paragraph also discusses how the structure of the halogenoalkane can affect whether substitution or elimination is favored, with primary halogenoalkanes倾向于substitution and tertiary halogenoalkanes倾向于elimination.
💧 Elimination Reactions from Halogenoalkanes and Alcohols
This paragraph examines elimination reactions, both from halogenoalkanes with potassium hydroxide in ethanol and from alcohols with concentrated sulfuric acid or aluminum oxide as a catalyst. It describes the step-by-step mechanism of elimination from halogenoalkanes, including the base-catalyzed removal of a hydrogen atom and the formation of an alkene. The paragraph also touches on the potential for mixtures of alkenes to form and how they can be separated by fractional distillation.
🔄 Isomerism in Elimination Reactions
The final paragraph wraps up the discussion on organic mechanisms by addressing isomerism that can result from elimination reactions. It uses butane as an example to illustrate how different pathways can lead to the formation of isomeric alkenes, such as But-1-ene and But-2-ene, and their respective Z and E isomers. The paragraph concludes by summarizing the key points covered in the video script, aiming to provide a comprehensive understanding of year one organic chemistry mechanisms.
Mindmap
Keywords
💡Electrophilic Addition
💡Nucleophilic Substitution
💡Carbocation
💡Electron Movement
💡Major and Minor Products
💡Nucleophile
💡Elimination Reaction
💡Halogenoalkanes
💡Alkenes
💡Curly Arrows
Highlights
Introduction to major organic mechanisms in year one chemistry, including electrophilic addition, nucleophilic substitution, and elimination.
Explanation of how to use curly arrows to represent the movement of electrons in chemical reaction mechanisms.
Description of electrophilic addition reactions involving alkenes and common electrophiles like hydrogen halides, bromine, and sulfuric acid.
Mechanism of ethene reacting with hydrogen bromide, illustrating the formation of a carbocation intermediate.
Discussion on the stability of carbocations and how it affects the major and minor products formed in electrophilic addition.
Explanation of nucleophilic substitution involving halogenoalkanes and nucleophiles such as hydroxide, cyanide, and ammonia.
Mechanism of cyanide ion attacking a halogenoalkane, leading to the formation of a new bond and the departure of a halide ion.
Differences in mechanism when ammonia is used as a nucleophile, resulting in the formation of amines and ammonium salts.
The importance of carbocation stability in determining the major product in nucleophilic substitution reactions.
Introduction to elimination reactions, including conditions that favor substitution versus elimination.
Mechanism of elimination from halogenoalkanes using potassium hydroxide in ethanol, resulting in alkene and hydrogen halide formation.
The role of the solvent in determining whether the hydroxide ion acts as a base in elimination or a nucleophile in substitution.
Explanation of elimination from alcohols using concentrated sulfuric acid or aluminum oxide as a catalyst.
Production of different alkene isomers through elimination reactions, including the formation of E and Z isomers.
The impact of halogenoalkane structure on the倾向 of the reaction towards substitution or elimination.
Summary of the conditions and mechanisms for different types of organic reactions covered in the video.
Transcripts
Browse More Related Video
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement

Chem 51A 11/09/09 Ch. 6. Introduction to Understanding Organic Reactions

Intro to Orgo Mechanisms Nucleophilic Attack and Loss of Leaving Group

Nucleophiles and Electrophiles: Crash Course Organic Chemistry #12

Alkene Reactions

E1 and E2 Reactions: Crash Course Organic Chemistry #22
5.0 / 5 (0 votes)
Thanks for rating: