Reactivity Series Trick
TLDRThis educational video introduces a memorable mnemonic, 'Please Stop Calling Me A Zebra I Truly Like Her Calling Me Super Powerful Giraffe,' to help students recall the reactivity series of metals. It explains how metals are ranked from most to least reactive, with potassium at the top and gold at the bottom, and clarifies hydrogen's inclusion due to its electropositive nature. The video also illustrates how the series predicts single displacement reactions, using examples with zinc and silver reacting with hydrochloric acid. It encourages viewers to engage with the content by sharing mnemonics and subscribing to the channel for more educational content.
Takeaways
- 📚 The video introduces a mnemonic to remember the reactivity series of metals.
- 🔍 The mnemonic is 'Please Stop Calling Me A Zebra I Truly Like Her Calling Me Super Powerful Giraffe'.
- 🧠 The mnemonic acronym stands for Potassium, Sodium, Calcium, Magnesium, Aluminium, Zinc, Iron, Tin, Lead, Hydrogen, Copper, Mercury, Silver, Platinum, and Gold.
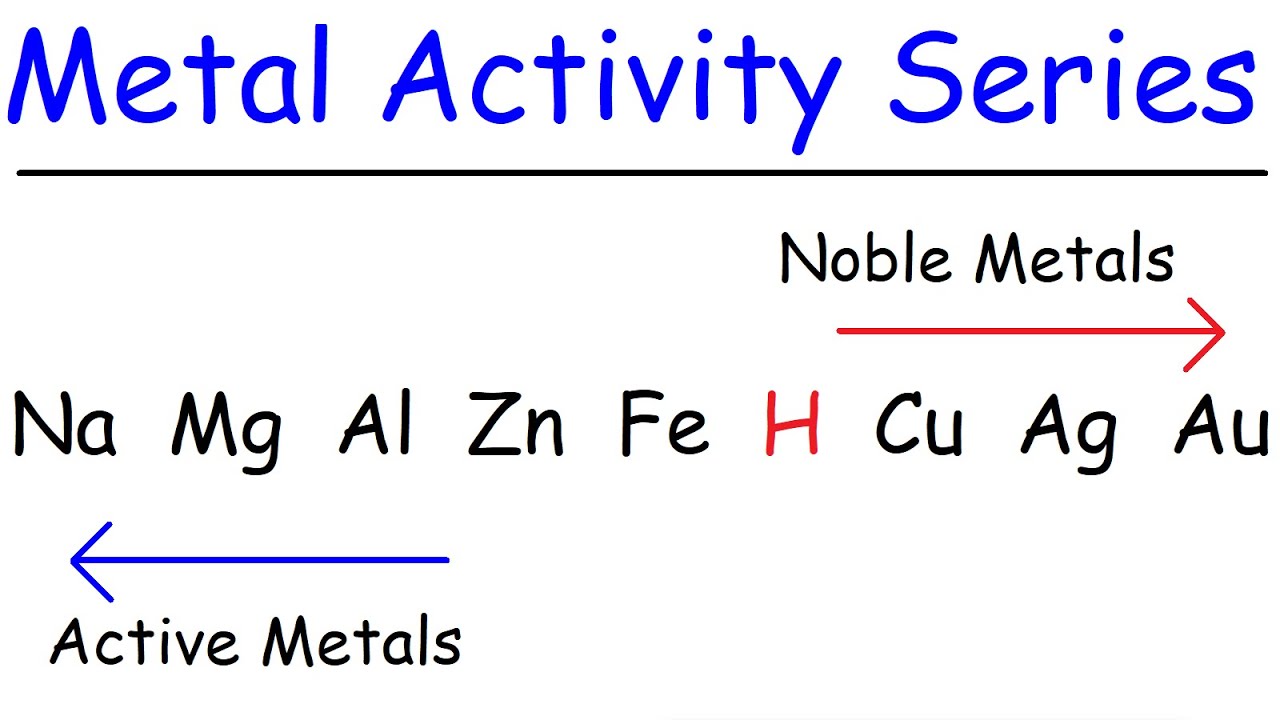
- 📉 Reactivity decreases down the list, with Potassium being the most reactive and Gold being the least reactive.
- 🤔 There is a note of variation where Platinum might be listed as the least reactive instead of Gold.
- 💡 Hydrogen is included in the series despite being a non-metal due to its electropositive nature and chemical properties similar to metals.
- 🔄 The reactivity series is used to predict single displacement reactions.
- ⚠️ In a single displacement reaction, a more reactive element can displace a less reactive one.
- 🌰 An example given is zinc displacing hydrogen in dilute hydrochloric acid to form zinc chloride and hydrogen gas.
- ❌ Silver does not react with dilute hydrochloric acid because it is less reactive than hydrogen.
- 📢 The video encourages viewers to subscribe to the channel and check out Manoj Academy's courses on their website.
Q & A
What is the purpose of the mnemonic in the video?
-The mnemonic helps remember the reactivity series of metals in order of their reactivity.
Which element is represented by 'please' in the mnemonic?
-Potassium.
What is the reactivity trend in the series mentioned in the video?
-Reactivity decreases as you move down the list from top to bottom.
Why is hydrogen included in the reactivity series of metals?
-Hydrogen has an electropositive nature and exhibits some chemical properties similar to metals, allowing it to be included in the series.
What reaction occurs when zinc reacts with dilute hydrochloric acid, according to the series?
-Zinc displaces hydrogen to form zinc chloride and hydrogen gas because zinc is more reactive than hydrogen.
Why does silver not react with dilute hydrochloric acid?
-Silver is less reactive than hydrogen and therefore cannot displace it in a reaction.
What variations in the reactivity series might you find in different books?
-Some books may list platinum as the least reactive metal instead of gold.
How is the reactivity series useful in predicting chemical reactions?
-It helps predict single displacement reactions by showing which metals can displace others based on their reactivity.
What does the mnemonic 'please stop calling me a zebra I truly like her calling me super powerful giraffe' represent?
-It represents the metals in the reactivity series in decreasing order of reactivity: Potassium, Sodium, Calcium, Magnesium, Aluminium, Zinc, Iron, Tin, Lead, Hydrogen, Copper, Mercury, Silver, Platinum, and Gold.
Why is the reactivity series important in chemistry?
-It helps understand the chemical behavior of metals, including predicting their reactions with other substances and understanding their displacement capabilities.
Outlines
🔬 Introduction to Reactivity Series Mnemonic
This paragraph introduces a memorable mnemonic to help students learn the reactivity series of metals. It explains that metals are arranged from most to least reactive and presents the mnemonic 'Please Stop Calling Me A Zebra I Truly Like Her Calling Me Super Powerful Giraffe' as a tool to remember the order, with each letter representing a metal. The paragraph also discusses the presence of hydrogen in the series due to its electropositive nature and chemical properties similar to metals. It emphasizes the practical use of the reactivity series in predicting single displacement reactions, where a more reactive metal displaces a less reactive one.
Mindmap
Keywords
💡Reactivity Series
💡Mnemonic
💡Potassium
💡Gold
💡Displacement Reaction
💡Hydrogen
💡Zinc
💡Silver
💡Chemical Properties
💡Electropositive Nature
💡Manoj Academy
Highlights
Introduction to learning the reactivity series of metals using a memorable mnemonic.
Explanation of the reactivity series, with highly reactive metals at the top and less reactive at the bottom.
Mnemonic 'Please Stop Calling Me A Zebra I Truly Like Her Calling Me Super Powerful Giraffe' for remembering the reactivity series.
Each word in the mnemonic corresponds to a metal in the reactivity series.
Potassium is the most reactive metal, while gold is the least.
Occasional variations in the reactivity series, such as platinum being listed as the least reactive instead of gold.
Inclusion of hydrogen in the reactivity series due to its electropositive nature and chemical properties similar to metals.
The reactivity series is used to predict single displacement reactions.
Only more reactive elements can displace less reactive ones in reactions.
Example of zinc displacing hydrogen in a reaction with dilute hydrochloric acid.
No reaction occurs when silver reacts with dilute hydrochloric acid due to its position below hydrogen in the reactivity series.
Encouragement for viewers to share their own mnemonics in the comments.
Request for likes, shares, and subscriptions to the YouTube channel.
Mention of a Hindi channel called 'Manoj Academy Hindi' for additional content.
Promotion of full courses available on manojacademy.com for various subjects.
Invitation to stay connected with Manoj Academy for ongoing learning.
The transcript concludes with a reminder to check out the provided links for further learning resources.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: