BTEC Applied Science: Unit 1 Chemistry Reactions of Metals
TLDRThis educational video script delves into the chemistry of metal reactions, focusing on three primary types: metal with oxygen forming metal oxides, metal with water producing metal hydroxides and hydrogen, and metal with dilute acids resulting in salts and hydrogen. It explains the concept of reactivity series, highlighting how more reactive metals displace less reactive ones in reactions, and touches on the trends in reactivity, such as the influence of the metal's position relative to the nucleus on its reactivity. The script also poses questions to engage viewers in applying their knowledge to predict outcomes of specific reactions.
Takeaways
- 🔍 Introduction to three main types of chemical reactions involving metals: with oxygen, water, and dilute acids.
- 🌟 Metal plus oxygen reaction leads to the formation of metal oxide. For instance, magnesium reacts with air to form magnesium oxide.
- 🛡️ Aluminium forms a protective layer of aluminium oxide, which prevents it from corroding quickly in the air.
- 📈 Reactivity of metals with oxygen varies; less reactive metals like silver tarnish over time due to oxidation.
- 💧 Reaction of metals with water can produce metal hydroxide and hydrogen gas, with more reactive metals like lithium, sodium, and potassium producing an alkaline solution.
- 🧪 Group 1 and Group 2 metals react directly with water, while less reactive metals like iron react slowly, leading to rust.
- 🔄 Metal plus dilute acid reactions result in the formation of salt and hydrogen gas due to displacement of hydrogen by the metal, which is more reactive.
- 📊 The reactivity series of metals helps predict how metals will react with acids, with more reactive metals displacing less reactive ones.
- 🔄 Understanding displacement reactions is crucial, as it involves the more reactive metal taking the place of hydrogen in the acid.
- 🚀 Reactivity trends: Metals are more reactive if their outer electrons are easier to remove, influenced by their distance from the nucleus and the shielding effect of inner electron shells.
- 🌈 Predicting reaction outcomes: Knowing the reactivity series and the nature of the reactions allows one to predict the products and behavior of different metal reactions.
Q & A
What are the three types of reactions involving metals discussed in the transcript?
-The three types of reactions involving metals discussed are: metal with oxygen forming metal oxide, metal with water forming metal hydroxide and hydrogen, and metal with dilute acid forming salt and hydrogen.
What happens when magnesium reacts with oxygen?
-When magnesium reacts with oxygen, magnesium oxide is formed.
How does aluminum protect itself from corrosion in the air?
-Aluminum forms a layer of aluminum oxide on its surface, which helps protect the metal underneath from corrosion.
Why do reactive metals like lithium, sodium, and potassium form an alkaline solution when they react with water?
-Reactive metals like lithium, sodium, and potassium react with water to form metal hydroxides, which dissolve in water and result in an alkaline solution.
What is the role of hydrogen in the context of metal and acid reactions?
-In the context of metal and acid reactions, hydrogen acts as a placeholder in the reactivity series. Metals that are more reactive than hydrogen can displace hydrogen from the acid, forming a salt and releasing hydrogen gas.
What is a salt in the context of chemical reactions?
-A salt in the context of chemical reactions is a compound that forms when an acid is neutralized.
What happens when sodium is dropped into water?
-When sodium is dropped into water, it reacts vigorously, producing hydrogen gas and sodium hydroxide, resulting in an alkaline solution.
What would be the balanced chemical equation for the reaction of sodium with water?
-The balanced chemical equation for sodium reacting with water is: 2Na + 2H2O → 2NaOH + H2↑.
What color change would be observed if universal indicator is added to the water when sodium reacts?
-If universal indicator is added to the water when sodium reacts, the solution would turn blue due to its alkaline nature.
Why is sodium more reactive than lithium?
-Sodium is more reactive than lithium because it is further away from the nucleus, which results in a weaker attraction between the nucleus and the outermost electron. This makes it easier for sodium to lose its outer electron compared to lithium.
What trend in reactivity can be observed when moving from left to right across the periodic table?
-When moving from left to right across the periodic table, elements become less reactive. This is because there are more electrons to overcome, requiring more energy to remove the outermost electron.
Outlines
🌟 Chemistry of Metal Reactions
This paragraph introduces the chemistry behind the reactions of metals, focusing on three main types of reactions: metal with oxygen, metal with water, and metal with dilute acid. It explains that metal plus oxygen forms metal oxide, metal plus water yields metal hydroxide and hydrogen, and metal plus dilute acid results in a salt and hydrogen. The paragraph uses magnesium and aluminum as examples to illustrate how these reactions work in practice. It also discusses the reactivity series, highlighting that more reactive metals like lithium, sodium, and potassium produce an alkaline solution when reacting with water, while less reactive metals like iron react slowly and can rust. The concept of displacement reactions is introduced, where a more reactive metal displaces a less reactive one from its compound.
🔍 Trends in Metal Reactivity and Reactions
This paragraph delves into the trends of metal reactivity, explaining why certain metals are more reactive than others. It mentions that reactivity increases as you move down the group in the periodic table because the outer electrons are further from the nucleus and easier to lose. The paragraph also discusses the decrease in reactivity moving from left to right across the periodic table due to the increasing number of electron shells. The summary includes an explanation of how the attraction of the nucleus weakens with distance, making it easier to remove an electron. The paragraph concludes with a set of questions for the viewer to apply their understanding, such as predicting the outcome of sodium reacting with water and identifying the color change of a universal indicator in the presence of the resulting solution.
Mindmap
Keywords
💡Metal Oxides
💡Aluminium Oxide
💡Reactivity Series
💡Displacement Reactions
💡Metal Hydrides
💡Alkaline Solution
💡Salts
💡Corrosion
💡Tarnishing
💡Reactivity Trends
💡Universal Indicator
Highlights
Introduction to the chemistry reactions of metals, specifically focusing on three key reactions involving oxygen, water, and dilute acids.
Explanation of the reaction between metal and oxygen, leading to the formation of metal oxides, using magnesium and aluminum as examples.
Discussion on how reactive metals such as lithium, sodium, potassium, and aluminum interact with oxygen, and the protective oxide layer formed by aluminum.
Description of the tarnishing process of metals like lithium, sodium, and silver when they react with oxygen.
Explanation of the reaction between metals and water, resulting in metal hydroxides and the release of hydrogen gas, with a focus on the reactivity series of group 1 metals.
Mention of the alkaline solution produced when reactive metals from group 1 and 2 react with water, due to the formation of metal hydroxides.
Comparison of the reactivity of different metals with water, such as lithium being more reactive than sodium and potassium.
Explanation of the reaction between metals and dilute acids, leading to the formation of salts and hydrogen gas, with the salt depending on the metal and acid involved.
Clarification on what constitutes a salt in the context of acid neutralization and the displacement of hydrogen by more reactive metals.
Prediction of the products formed when specific metals react with different acids, such as sodium with hydrochloric acid and calcium with sulfuric acid.
Discussion on the trend of reactivity among metals, with more reactive metals being easier to strip of their outer electrons due to weaker nuclear attraction.
Explanation of why potassium is more reactive than sodium, relating to the distance from the nucleus and the shielding effect of inner electron shells.
Observation of the decrease in reactivity when moving from left to right in the periodic table due to the increasing number of electrons.
Introduction to the concept of displacement reactions, where a more reactive metal displaces a less reactive one from its compound.
Questions posed to the audience to engage them in applying the learned concepts, such as predicting the reaction of sodium with water and the resulting color change of a universal indicator.
Transcripts
Browse More Related Video

Reactions Of Metals With Water | Reactions | Chemistry | FuseSchool

GCSE Chemistry - Reactivity Series of Metals & Displacement Reactions #37

BTEC Applied Science: Unit 1 Chemistry Reactions with Oxygen

Predicting Products | Single Replacement Reactions

Reactivity series of metals
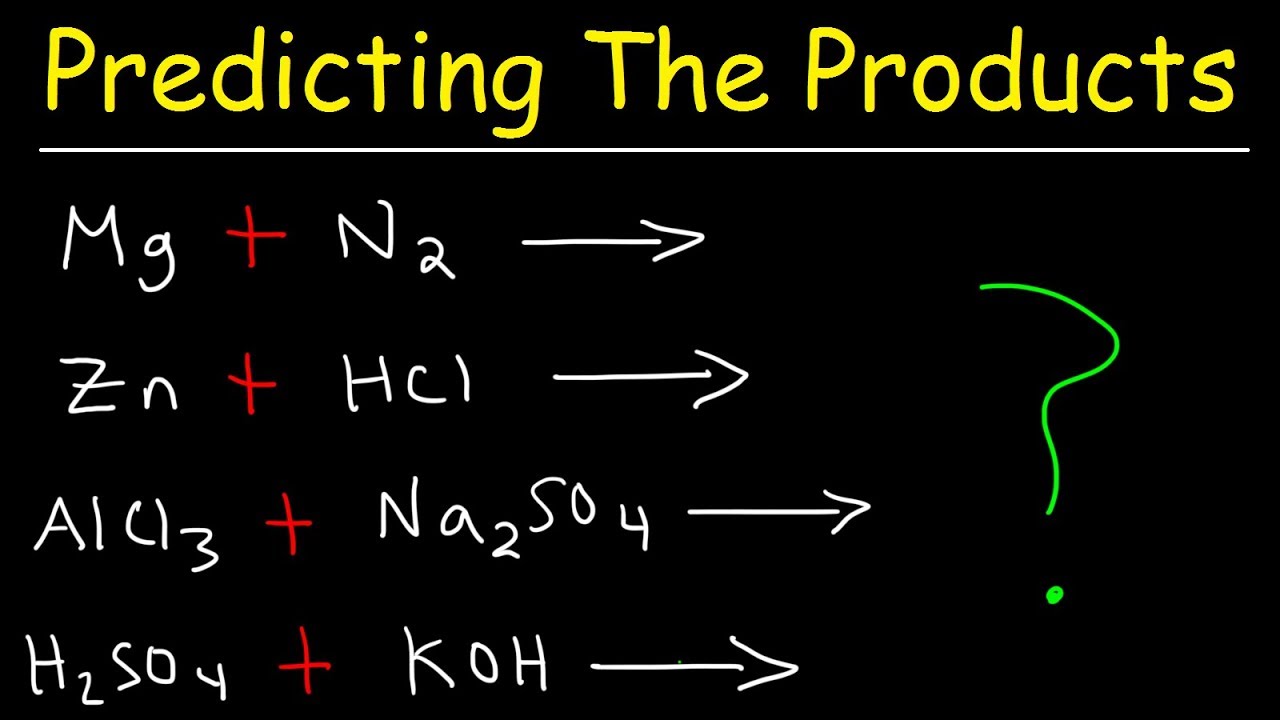
Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: