Chemistry - Will The Reaction Occur?
TLDRThis educational video script explains the concept of single displacement reactions using the activity series of metals and halogens. It illustrates how to determine if a reaction will proceed by comparing the reactivity of elements. The script provides examples, such as zinc displacing copper and aluminum reacting with hydrochloric acid, to demonstrate predicting products and balancing chemical equations. It also clarifies that iodine cannot displace chloride due to lower reactivity, offering a clear guide to understanding single replacement reactions.
Takeaways
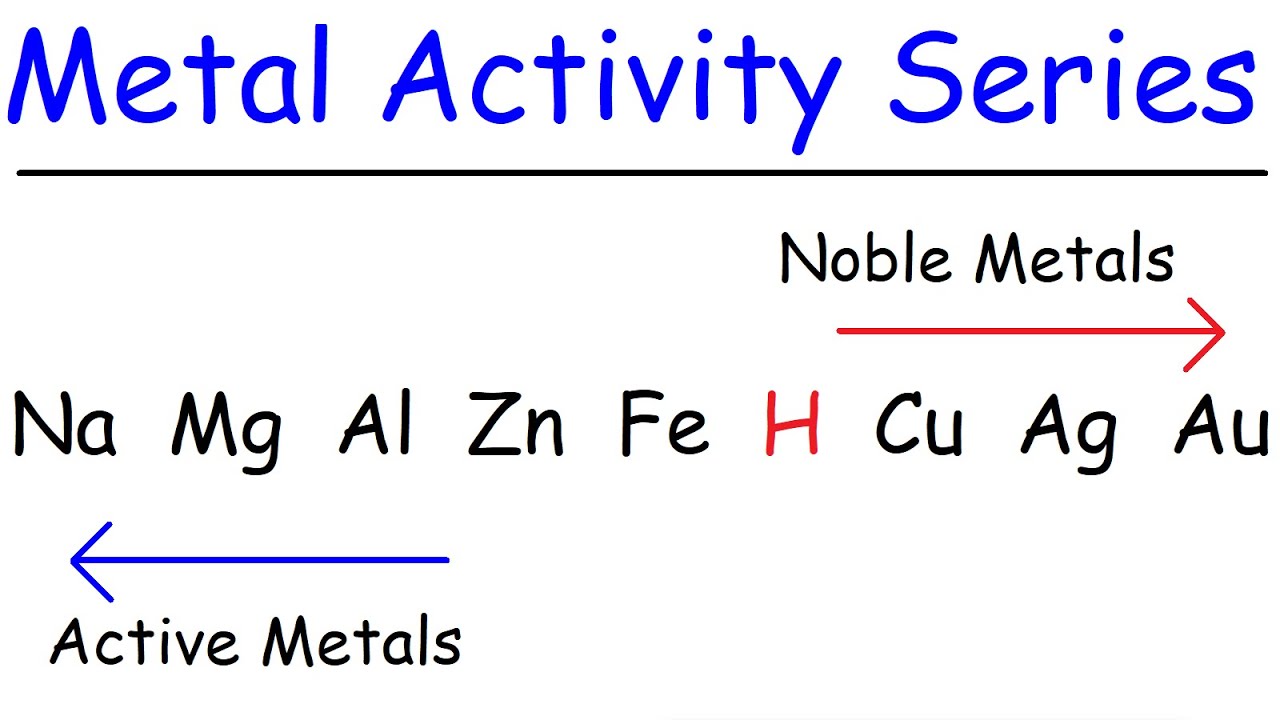
- 🔍 The activity series is a tool used to predict the outcome of single displacement reactions.
- 📊 Elements to the left in the activity series are more reactive, with lithium being the most reactive and gold and silver being the least.
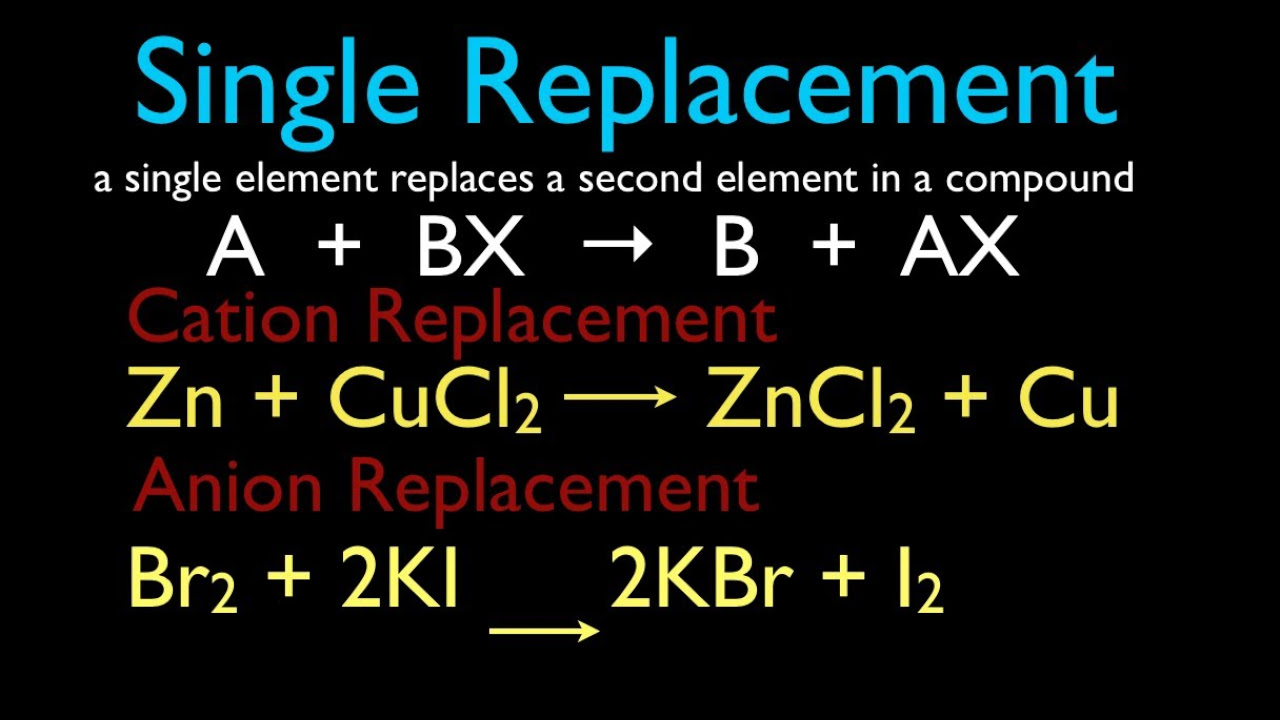
- 🧩 Zinc can displace copper in an aqueous copper II chloride solution because zinc is more reactive than copper.
- 📝 In a single displacement reaction, the more reactive element displaces a less reactive element from its compound.
- 🌐 The products of a reaction can be determined by knowing the reactivity of elements and balancing the charges in the resulting compounds.
- 💧 Aqueous solutions are indicated by 'aq' in chemical formulas, showing that the substance is soluble in water.
- ❌ Copper cannot displace iron in iron II sulfate because copper is less reactive than iron.
- 🌀 Aluminum can displace hydrogen in hydrochloric acid due to its higher reactivity, forming aluminum chloride and hydrogen gas.
- ⚖️ Balancing chemical equations involves adjusting coefficients to ensure equal numbers of atoms on both sides of the equation.
- 🌊 Halogens have their own activity series, with fluorine being the most reactive and iodine the least.
- ❌ Iodine cannot displace chloride ions in sodium chloride because iodine is less reactive than chlorine.
Q & A
What is a single displacement reaction?
-A single displacement reaction is a type of chemical reaction in which an element in a compound is replaced by another element, often resulting in the formation of a new element and a new compound.
What is the significance of the activity series in chemistry?
-The activity series is a list of metals arranged in order of decreasing reactivity. It is used to predict whether a metal will displace another metal in a compound, which is crucial for determining the feasibility of a single displacement reaction.
Why is zinc able to displace copper in an aqueous solution of copper II chloride?
-Zinc is able to displace copper in an aqueous solution of copper II chloride because zinc is positioned to the left of copper in the activity series, indicating that it is more reactive and can thus replace copper in the compound.
What are the products of the reaction between zinc and aqueous copper II chloride?
-The products of the reaction between zinc and aqueous copper II chloride are elemental copper, which precipitates out of the solution, and zinc chloride, which is soluble in water and remains in the aqueous phase.
How can you determine if copper metal will displace iron in iron II sulfate?
-Copper metal will not displace iron in iron II sulfate because copper is positioned to the right of iron in the activity series, indicating that it is less reactive and therefore cannot replace iron in the compound.
What happens when aluminum metal is mixed with aqueous hydrochloric acid?
-When aluminum metal is mixed with aqueous hydrochloric acid, a single displacement reaction occurs where aluminum, being more reactive than hydrogen, displaces hydrogen to form aluminum chloride and hydrogen gas.
Why is it important to balance charges when writing the formula of an ionic compound?
-Balancing charges is important to ensure that the total positive charge equals the total negative charge in an ionic compound, resulting in a neutral compound that can exist stably.
How do you balance the chemical equation for the reaction between aluminum and hydrochloric acid?
-To balance the chemical equation, you adjust the coefficients of the reactants and products so that the number of atoms of each element on both sides of the equation is equal, ensuring mass conservation.
What is the role of the halogens activity series in predicting reactions involving halogens?
-The halogens activity series, similar to the metal activity series, lists halogens in order of decreasing reactivity. It helps predict whether a halogen can displace another halogen from a compound in a single displacement reaction.
Why does the reaction between chlorine gas and aqueous sodium bromide proceed?
-The reaction between chlorine gas and aqueous sodium bromide proceeds because chlorine is more reactive than bromine according to the halogens activity series, allowing it to displace bromine and form sodium chloride and elemental bromine.
What is the outcome of the reaction between solid iodine and aqueous sodium chloride?
-The reaction between solid iodine and aqueous sodium chloride does not proceed because iodine is less reactive than chlorine, and thus it cannot displace the chloride ion from the solution.
Outlines
🔬 Understanding Single Displacement Reactions with the Activity Series
This paragraph introduces the concept of single displacement reactions and the use of the activity series to predict whether a reaction will occur. It explains that the reactivity of metals is crucial in determining the outcome of such reactions. The narrator provides a simplified list of elements in the activity series, emphasizing that lithium is the most reactive and gold and silver are the least. The paragraph uses the example of zinc reacting with copper II chloride to illustrate how to apply the activity series, concluding that zinc, being more reactive, will displace copper. The summary also touches on how to determine the products of the reaction, explaining the formation of zinc chloride (ZnCl2) and elemental copper.
🧪 Predicting Reaction Outcomes and Balancing Chemical Equations
The second paragraph delves deeper into predicting the outcomes of single displacement reactions and the process of balancing chemical equations. It uses the example of copper reacting with iron II sulfate to demonstrate that copper, being less reactive than iron, will not displace iron from the solution, resulting in no reaction. The paragraph then moves on to the reaction of aluminum with hydrochloric acid, showing that aluminum, being more reactive than hydrogen, will displace it to form aluminum chloride (AlCl3) and hydrogen gas (H2). The narrator guides through the process of balancing the chemical equation, emphasizing the importance of using whole numbers and achieving atom balance.
🌐 Activity Series Application in Halogens and Final Examples
The final paragraph extends the discussion to halogens, presenting a different activity series for these elements and explaining how a more reactive halogen can displace a less reactive one from a solution. The narrator uses the example of chlorine gas reacting with aqueous sodium bromide, predicting that chlorine, being more reactive, will displace bromine to form sodium chloride (NaCl) and elemental bromine (Br2). The paragraph concludes with an example involving iodine and aqueous sodium chloride, where iodine, being less reactive than chlorine, will not displace chloride ions, leading to no reaction. The summary highlights the importance of using the correct activity series for metals and halogens to predict reaction outcomes in single displacement reactions.
Mindmap
Keywords
💡Reactivity
💡Activity Series
💡Single Displacement Reaction
💡Zinc Metal
💡Aqueous Solution
💡Ionic Compound
💡Charge Balance
💡Halogens
💡Chemical Formula
💡Balancing Equations
💡No Reaction
Highlights
Introduction to the concept of the activity series in predicting the outcome of single displacement reactions.
Explanation of the activity series, with examples of metals and their reactivity from lithium to gold.
Demonstration of how to use the activity series to determine if a reaction will proceed, using zinc and copper as an example.
Description of the single displacement reaction between zinc metal and aqueous copper II chloride, leading to the formation of zinc chloride and copper metal.
Illustration of balancing charges in ionic compounds to write the correct formula, exemplified by ZnCl2.
Practice example involving copper metal and iron II sulfate, explaining why no reaction occurs due to copper being less reactive than iron.
Application of the activity series to halogens, with chlorine being more reactive than bromine, leading to a potential reaction.
Explanation of the reaction between chlorine gas and aqueous sodium bromide, resulting in the formation of elemental bromine and sodium chloride.
Guidance on balancing chemical equations using whole numbers to avoid fractions, as shown in the aluminum and hydrochloric acid example.
Discussion on the reactivity of metals with hydrogen in single displacement reactions, using aluminum as an example.
Prediction of the outcome of a reaction between iodine and aqueous sodium chloride, concluding no reaction due to iodine's lower reactivity.
Emphasis on the importance of using the activity series to determine the feasibility of single replacement reactions.
Advice on how to approach writing chemical reactions involving halogens, differentiating it from metal displacement reactions.
Final summary of the process for predicting single displacement reactions using the activity series and balancing chemical equations.
Encouragement for viewers to subscribe and explore the chemistry video playlist for further learning.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: