Conjugate Acid Base Pairs, Arrhenius, Bronsted Lowry and Lewis Definition - Chemistry
TLDRThis video script delves into the concepts of acids and bases, focusing on three key definitions: Arrhenius, Bronsted-Lowry, and Lewis. It explains how Arrhenius acids release H+ ions and bases release OH- ions in solution, while Bronsted-Lowry definitions involve proton donors and acceptors. The script further explores the formation of conjugate acids and bases, and concludes with the Lewis definition, highlighting electron pair acceptors and donors in chemical reactions. The examples provided illustrate these theories in a clear and engaging manner.
Takeaways
- 📚 The Arrhenius definition of acids involves the release of H+ ions or hydronium ions (H3O+) in solution.
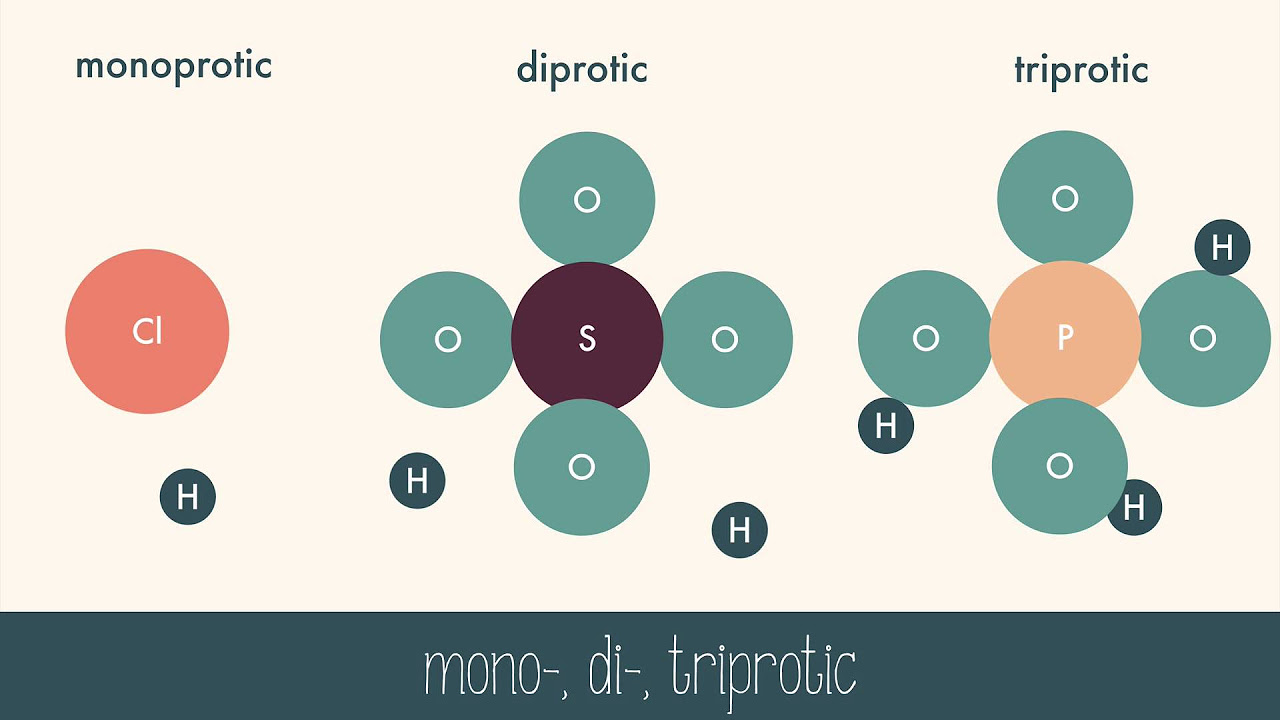
- 🧪 Common examples of Arrhenius acids include HF, HCl, H2SO4, and HNO3, all of which donate a hydrogen ion to form their respective conjugate bases and hydronium ions.
- 🥼 Arrhenius bases release hydroxide ions (OH-) in solution, causing an increase in pH and making the solution more basic or alkaline.
- 🔄 The Bronsted-Lowry definition expands the concept of acids and bases by defining them as proton donors and acceptors, respectively.
- 🌟 In a Bronsted-Lowry reaction, the conjugate acid is formed when a base accepts a proton, and the conjugate base is formed when an acid donates a proton.
- 📈 The process of finding conjugate acid-base pairs involves adjusting the number of hydrogen atoms and charges on the molecules or ions accordingly.
- 🤖 Lewis acids and bases are defined in terms of electron pairs, with Lewis acids accepting electron pairs and Lewis bases donating them.
- 🔌 The Lewis acid-base reaction involves the formation of a coordinate covalent bond, where the Lewis base donates a pair of electrons to the Lewis acid.
- 🌐 The concept of nucleophiles and electrophiles is central to understanding Lewis acid-base reactions, with nucleophiles being electron-rich and electrophiles being electron-poor.
- 📊 The script provides a comprehensive overview of acid-base theories, highlighting the differences between Arrhenius, Bronsted-Lowry, and Lewis definitions, and their practical applications in chemistry.
- 🔍 Understanding these definitions and concepts is crucial for predicting the behavior of substances in chemical reactions and their classification as acids or bases.
Q & A
What is the Arrhenius definition of an acid?
-The Arrhenius definition of an acid is a substance that releases H+ ions, also known as hydronium ions (H3O+), when dissolved in water.
How does an Arrhenius base behave in solution?
-An Arrhenius base releases hydroxide ions (OH-) in solution, which leads to an increase in pH, making the solution more basic or alkaline.
Give an example of an Arrhenius acid and explain its behavior in water.
-Hydrochloric acid (HCl) is an example of an Arrhenius acid. When dissolved in water, it dissociates to donate a hydrogen ion (H+) to water, forming hydronium ions (H3O+) and chloride ions (Cl-).
What is the difference between a Bronsted-Lowry acid and a Bronsted-Lowry base?
-A Bronsted-Lowry acid is a proton (H+) donor, while a Bronsted-Lowry base is a proton acceptor.
How does the reaction between hydrofluoric acid (HF) and water illustrate the Bronsted-Lowry definition?
-In the reaction, HF acts as a Bronsted-Lowry acid by donating a proton to water, forming hydronium ions (H3O+) and fluoride ions (F-). Water acts as a Bronsted-Lowry base by accepting the proton.
What is the conjugate acid of a bicarbonate ion (HCO3-)?
-The conjugate acid of a bicarbonate ion is carbonic acid (H2CO3), formed by adding one hydrogen atom to the bicarbonate ion and increasing its charge by one unit.
What is the conjugate base of the bicarbonate ion (HCO3-)?
-The conjugate base of a bicarbonate ion is the carbonate ion (CO3^2-), formed by removing one hydrogen atom from the bicarbonate ion and decreasing its charge by one unit.
How does the Lewis definition of acids and bases differ from the Bronsted-Lowry definition?
-The Lewis definition of acids and bases involves electron pairs. A Lewis acid is an electron pair acceptor, while a Lewis base is an electron pair donor.
What is an example of a Lewis acid-base reaction and how does it differ from an Arrhenius or Bronsted-Lowry reaction?
-An example of a Lewis acid-base reaction is the reaction between BH3 and ammonia (NH3). In this reaction, ammonia donates a pair of electrons to boron, making it the Lewis base, while boron accepts the electrons, making it the Lewis acid. This differs from Arrhenius and Bronsted-Lowry reactions, which involve the transfer of protons (H+).
What are the roles of the nucleophile and electrophile in a Lewis acid-base reaction?
-In a Lewis acid-base reaction, the nucleophile is the electron-rich species that donates a pair of electrons, typically the Lewis base. The electrophile is the electron-poor species that accepts the electron pair, typically the Lewis acid.
How can you determine the conjugate acid and base of a given molecule or ion?
-To determine the conjugate acid, increase the hydrogen number by one and adjust the charge accordingly. To find the conjugate base, remove a hydrogen and adjust the charge to reflect the change.
Outlines
📚 Introduction to Acids and Bases - Theories and Definitions
This paragraph introduces the viewer to the fundamental concepts of acids and bases, focusing on three main definitions: Arrhenius, Bronsted-Lowry, and Lewis. The Arrhenius definition describes acids as substances that release H+ ions (or hydronium ions, H3O+) in solution and bases as those that release hydroxide ions (OH-). The paragraph provides examples of Arrhenius acids such as HF, HCl, H2SO4, and HNO3, and bases like sodium hydroxide, potassium hydroxide, and calcium hydroxide. It also explains the behavior of these substances in water, highlighting the formation of hydronium ions from H+ and water and the generation of conjugate bases. The Bronsted-Lowry definition is introduced as a broader concept, defining acids as proton donors and bases as proton acceptors, with an example reaction between hydrofluoric acid (HF) and water to illustrate these roles. The paragraph concludes with an explanation of how to identify conjugate acids and bases, using the bicarbonate ion (HCO3-) as an example.
🧪 Further Exploration of Acids and Bases - Conjugate Pairs and Lewis Theory
This paragraph delves deeper into the concept of conjugate acid-base pairs, providing a method for determining the conjugate acid and base for various molecules and ions. It explains the process of increasing the hydrogen count by one and adjusting the charge to find the conjugate acid, and decreasing the hydrogen count by one and adjusting the charge to find the conjugate base. The paragraph uses examples such as ammonia (NH3) and the bicarbonate ion (HCO3-) to illustrate these concepts. It then revisits the Bronsted-Lowry definition, using the reaction between carbonate and water to demonstrate the identification of the acid and base in a reaction, and the formation of conjugate acid and base pairs. The paragraph concludes with an introduction to the Lewis definition of acids and bases, where acids are electron pair acceptors and bases are electron pair donors, using the reaction between BH3 and ammonia as an illustrative example.
🌟 Lewis Acid-Base Theory and the Concept of Electron Pairs
The final paragraph of the script focuses on the Lewis acid-base theory, which expands upon the concept of acids and bases as electron pair acceptors and donors, respectively. It explains that a Lewis acid is an electron pair acceptor, while a Lewis base is an electron pair donor. The paragraph uses the reaction between boron hydride (BH3) and ammonia (NH3) to illustrate this theory. In this reaction, the nitrogen atom in ammonia has a lone pair of electrons that it can donate, making it a Lewis base, while boron, with an incomplete octet, can accept this pair of electrons, making it a Lewis acid. The formation of a bond between boron and nitrogen results in a product where nitrogen has a positive formal charge (due to four bonds) and boron has a negative formal charge (with four bonds), maintaining overall charge neutrality. The paragraph highlights the roles of nucleophiles (electron-rich species that donate electron pairs) and electrophiles (electron-poor species that accept electron pairs) in Lewis acid-base reactions.
Mindmap
Keywords
💡Acids
💡Bases
💡Arrhenius Definition
💡Bronsted-Lowry Definition
💡Conjugate Acid-Base Pairs
💡Hydronium Ion
💡Protons
💡Lewis Acid-Base Definition
💡Nucleophile
💡Electrophile
💡pH
Highlights
The Arrhenius definition of acids is that they release H+ ions or hydronium ions in solution.
In water, H+ ions associate with water to produce the hydronium ion (H3O+).
Arrhenius bases release hydroxide ions (OH-) in solution, which increases the pH and makes the solution basic or alkaline.
Examples of Arrhenius acids include HF, HCl, H2SO4, and HNO3, all of which have a hydrogen atom in front of them.
Hydrochloric acid (HCl) dissociates in water, donating a hydrogen to form the hydronium ion and the conjugate base chloride.
A Bronsted-Lowry acid is a proton donor, and a Bronsted-Lowry base is a proton acceptor.
Hydrofluoric acid (HF) is considered both an Arrhenius acid and a Bronsted-Lowry acid because it donates a proton to water.
Water can act as both a Bronsted-Lowry acid and a base, depending on the reaction.
The conjugate acid is formed by adding a hydrogen to a molecule, and the conjugate base is formed by removing a hydrogen.
The bicarbonate ion (HCO3-) has a conjugate acid (H2CO3) and a conjugate base (CO3 2-) based on the number of hydrogens and charge.
Ammonia (NH3) acts as a Bronsted-Lowry base by accepting a hydrogen ion to become NH4+, which is the conjugate acid.
The monohydrogen phosphate ion (HPO4 2-) has a conjugate acid (H2PO4-) and a conjugate base (PO4 3-) based on the change in hydrogen number and charge.
The Bronsted-Lowry definition helps identify the acid and base in a reaction by looking at proton transfer.
Carbonates (CO3 2-) can act as bases by accepting hydrogen ions to become bicarbonate ions (HCO3-), with water acting as the acid.
Lewis acids are electron pair acceptors, and Lewis bases are electron pair donors.
An example of a Lewis acid-base reaction is the interaction between BH3 and ammonia, where ammonia donates a lone pair of electrons to boron.
In Lewis acid-base reactions, the base (nucleophile) is electron-rich, and the acid (electrophile) is electron-poor.
Transcripts
Browse More Related Video

Introduction to Acid-Base Chemistry - AP Chemistry Unit 4, Topic 8

3.1 Introduction to Acids and Bases | Organic Chemistry

What Is The Bronsted Lowry Theory | Acids, Bases & Alkali's | Chemistry | FuseSchool

Acid Base Introduction
Acid-Base Theories

16.1 Introduction to Acids and Bases | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: