Know This For Your Chemistry Final Exam - Stoichiometry Review
TLDRThe video script is a comprehensive guide to understanding stoichiometry, a fundamental concept in chemistry. It introduces four main conversion factors: molar mass, multiple ratio, Avogadro's number, and STP (standard temperature and pressure). The script emphasizes the importance of these factors in converting between different units, such as grams to moles or moles to molecules. The process is likened to solving puzzles, where each conversion factor is a piece that helps to connect and cancel out units to reach the desired unit. The guide provides step-by-step instructions on how to approach stoichiometry problems, starting with the given information, converting to moles, using multiple ratios to change compounds, and finally converting back to the required unit. The script also encourages practice and offers additional resources for a deeper understanding of stoichiometry.
Takeaways
- 📚 **Stoichiometry Puzzles:** Stoichiometry problems are like puzzles where conversion factors are the pieces that help you connect different units.
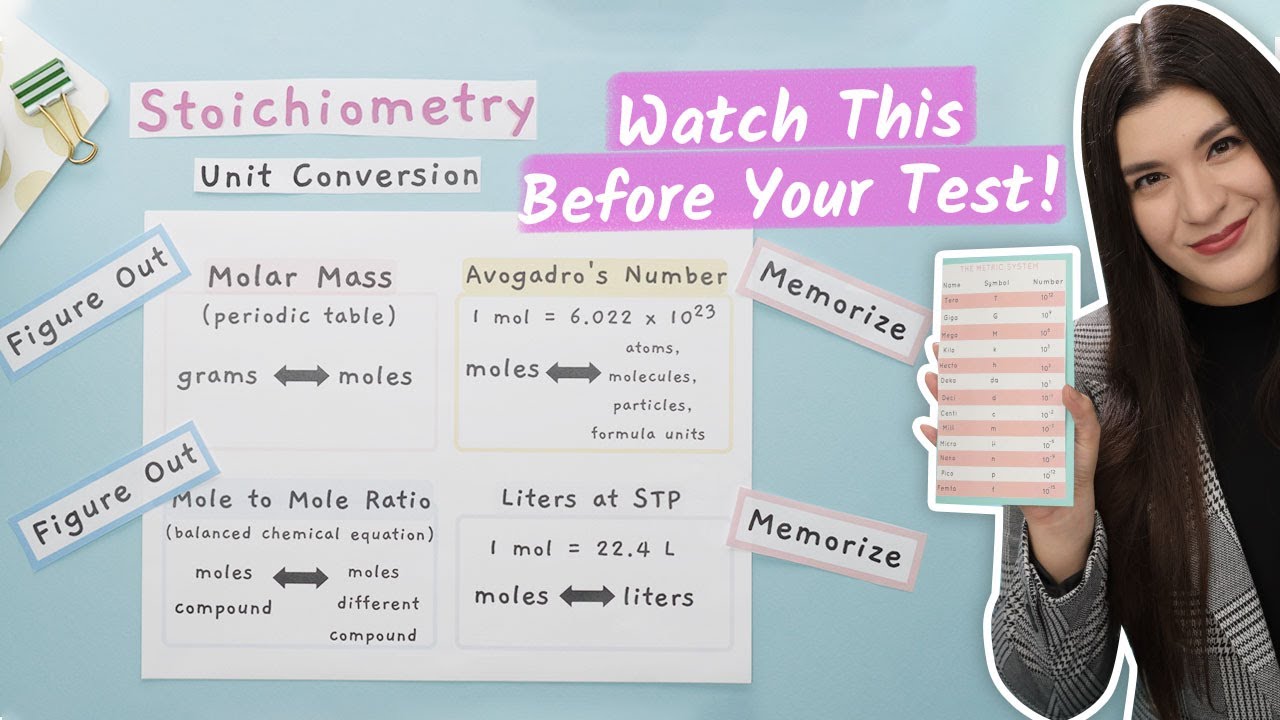
- 🔍 **Molar Mass:** Molar mass is the first conversion factor that allows you to convert between grams and moles of a substance.
- 🔄 **Multiple Ratio:** The multiple ratio is used to change compounds, derived from balanced equations or subscripts in compounds, and helps to find mole to mole ratios.
- 🧬 **Avogadro's Number:** Avogadro's number (6.02 x 10^23) is used to convert between moles and the number of atoms, molecules, or particles.
- 🌡️ **STP Conversion:** At standard temperature and pressure (STP), one mole of a gas occupies 22.4 liters, which is another conversion factor used in stoichiometry.
- ↔️ **Unit Cancellation:** Align units correctly to cancel them out, which guides you to the desired unit in a stoichiometry problem.
- 🔬 **Starting with Moles:** If unsure where to start, convert the given value to moles, as most conversion factors lead to moles.
- ⚖️ **Molar Mass for Conversion:** Use molar mass to convert between grams and moles, and vice versa.
- 🔄 **Multiple Ratio Application:** Apply the multiple ratio to change from one compound to another, guided by coefficients in a balanced chemical equation or subscripts in the compound formula.
- 📉 **Limiting Reactants:** The concept of limiting reactants is not covered in this script but is a crucial topic in stoichiometry, which will be covered in a subsequent video.
- 💡 **Understanding Patterns:** Recognizing patterns in stoichiometry, such as the sequence of conversions and unit alignment, is key to solving problems efficiently.
Q & A
What are the four main conversion factors used in stoichiometry?
-The four main conversion factors in stoichiometry are molar mass, multiple ratio, Avogadro's number, and liters at STP (standard temperature and pressure).
How does molar mass help in stoichiometry calculations?
-Molar mass allows you to convert between grams and moles of a substance, or vice versa, by providing a direct relationship between the mass of a substance and the number of molecules it contains.
What is a multiple ratio and how is it used in stoichiometry?
-A multiple ratio is used to convert moles of one compound to moles of a different compound. It is derived from the balanced chemical equation and can also be determined from the subscripts in the chemical formula of a compound.
What role does Avogadro's number play in stoichiometry?
-Avogadro's number, which is approximately 6.02 x 10^23, is used to convert between moles and the number of individual atoms, molecules, particles, or formula units in a sample.
How is STP (standard temperature and pressure) used in stoichiometry?
-At STP, one mole of any ideal gas occupies 22.4 liters. This relationship is used to convert between moles of a gas and the volume of the gas at standard conditions.
What is the first step when you are unsure of how to proceed with a stoichiometry problem?
-When unsure, the first step is to convert the given value to moles, as most stoichiometry problems involve moles at some point and this conversion helps to establish a path forward.
How do you determine the multiple ratio from a balanced chemical equation?
-The multiple ratio is determined from the coefficients in the balanced chemical equation. If there is no coefficient in front of a compound, it is assumed to be 1. The subscripts in the chemical formula also represent the multiple ratio for the atoms of that element within the compound.
Why is it important to align units correctly when performing stoichiometry calculations?
-Aligning units correctly ensures that the units cancel out appropriately during the calculation, leading to the desired unit for the final answer. This step is crucial for maintaining accuracy in stoichiometry problems.
What is the significance of the diatomic nature of oxygen in stoichiometry?
-Oxygen is diatomic, meaning it exists as O2 rather than O when in its standard state. This is significant because it affects the multiple ratio and the number of moles of oxygen atoms when converting between different compounds.
How can you identify when to use the conversion factor of molar mass in a stoichiometry problem?
-You use the conversion factor of molar mass when you need to convert between grams and moles of a substance. It is often the first or last step in a stoichiometry problem, depending on whether you are starting with grams and need moles, or ending with moles and need grams.
What is the purpose of practicing stoichiometry problems?
-Practicing stoichiometry problems helps to solidify understanding of the concepts and improve problem-solving skills. It is a fundamental part of chemistry, and mastering these problems is essential for success in the subject.
Where can one find additional resources for learning stoichiometry?
-Additional resources for learning stoichiometry can be found on Melissa Maribel's website, where a complete guide to stoichiometry with more examples and practice test questions is available for purchase.
Outlines
🔍 Understanding Stoichiometry Basics
This paragraph introduces the concept of stoichiometry and its application in chemistry, particularly for a YouTuber named Selena Sanchez preparing for her final exam. It emphasizes the importance of conversion factors in stoichiometry, which act as puzzle pieces to connect different units. The paragraph outlines four main conversion factors: molar mass (grams to moles conversion), multiple ratio (changing compounds using mole ratios from balanced equations), Avogadro's number (moles to atoms, molecules, etc.), and liters at STP (moles to volume at standard temperature and pressure). The focus is on how these factors help in solving stoichiometry problems by canceling out units to reach the desired unit.
🧩 Applying Conversion Factors in Stoichiometry
The second paragraph delves into the application of conversion factors in solving stoichiometry problems. It explains that stoichiometry is about canceling out units to obtain the desired unit. The paragraph provides a step-by-step approach to solving a problem involving converting grams of carbon monoxide to molecules of oxygen. It highlights the process of starting with the given information, identifying what is being asked (finding molecules of oxygen), and using molar mass to convert grams to moles. The paragraph also discusses the use of the multiple ratio from a balanced chemical equation to change compounds and Avogadro's number to convert moles to molecules, ultimately solving the stoichiometry problem.
📚 Advanced Stoichiometry Problem-Solving
The third paragraph continues the discussion on stoichiometry, focusing on more complex problem-solving. It guides the user through converting grams to moles using molar mass, then using a multiple ratio to change compounds, and finally converting moles back to grams. The paragraph clarifies the process of aligning units to cancel them out effectively. It also emphasizes the importance of stoichiometry in chemistry, likening it to the heart of the subject. The speaker offers additional resources for further practice and understanding, including a complete guide to stoichiometry with more examples and practice test questions, available for purchase.
Mindmap
Keywords
💡Stoichiometry
💡Conversion Factors
💡Molar Mass
💡Multiple Ratio
💡Avogadro's Number
💡Standard Temperature and Pressure (STP)
💡Balanced Equation
💡Diatomic Molecule
💡Limiting Reactants
💡Coefficients
💡Subscripts
Highlights
Stoichiometry is introduced as a set of puzzles where conversion factors are the pieces.
Molar mass is the first conversion factor, allowing the conversion between grams and moles.
Multiple ratio is used to change compounds, derived from balanced equations.
Avogadro's number is the third conversion factor, connecting moles to atoms, molecules, and particles.
Standard Temperature and Pressure (STP) is the fourth conversion factor, relating moles to liters at STP.
The process of stoichiometry involves canceling units to reach the desired unit.
When stuck, converting to moles is a safe starting step in a stoichiometry problem.
Multiple ratio is found in balanced equations and subscripts of compounds.
A demonstration of converting from grams of CO to moles of O2 using the four conversion factors.
Oxygen is always a diatomic molecule, existing in pairs as O2.
The importance of aligning units correctly to ensure they cancel out during stoichiometric calculations.
An example problem is solved, starting with grams of a compound and aiming to find molecules of another.
The concept of molar mass is used to convert grams to moles and vice versa.
A practice problem is presented for the viewer to solve, involving conversion from grams to grams of a different compound.
The periodic table is referenced for atomic masses needed in molar mass calculations.
The final step in an example problem involves converting moles back to grams using molar mass.
Stoichiometry is described as the heart of chemistry, emphasizing its importance in the subject.
The video concludes with a promotion for a complete guide to stoichiometry with more examples and practice test questions.
Transcripts
Browse More Related Video
How to Convert Units in Chemistry

Step by Step Stoichiometry Practice Problems | How to Pass Chemistry

Finding Grams and Liters Using Molarity - Final Exam Review

Avogadro's Number, The Mole, Grams, Atoms, Molar Mass Calculations - Introduction

Gas Stoichiometry: Equations Part 1

Converting Between Grams and Moles
5.0 / 5 (0 votes)
Thanks for rating: