Converting Between Grams and Moles
TLDRThe video script offers a comprehensive guide on converting between grams and moles, a fundamental concept in chemistry. It begins by explaining the molar mass of aluminum and how to calculate the mass in grams for a given number of moles. The script then illustrates two methods for this conversion: a straightforward multiplication approach and a conversion factor method. The same principles are applied to determine the number of moles in a given mass of sodium chloride (NaCl). The video emphasizes the importance of understanding molar mass and significant figures in calculations. It concludes with an encouragement to practice these conversions with more problems, suggesting a follow-up video for additional practice.
Takeaways
- 🧪 The molar mass of aluminum is 26.98 grams per mole, which means one mole of aluminum weighs 26.98 grams.
- 🔢 To convert moles to grams for aluminum, multiply the number of moles by the molar mass (e.g., 4.30 moles * 26.98 g/mole = 116 grams).
- 🔄 The concept of molar mass allows for the conversion between moles and grams by simple multiplication or division.
- 📚 Looking up the molar mass of an element on the periodic table provides the mass of one mole of that element.
- ⚖️ For sodium chloride (NaCl), the molar mass is calculated by adding the molar masses of sodium (Na) and chlorine (Cl) from the periodic table.
- 🧮 The molar mass of NaCl is 58.44 grams per mole, indicating that one mole of NaCl weighs 58.44 grams.
- ➗ To convert grams to moles, divide the mass in grams by the molar mass of the substance (e.g., 127.5 g / 58.44 g/mole ≈ 2.18 moles).
- 📉 Significant figures in calculations should be considered, rounding the final answer to maintain the same level of precision as the initial values.
- 📏 Both the direct thinking method and the conversion factor method result in the same mathematical operations for converting between moles and grams.
- 🔗 Conversion factors are based on the relationship between moles and mass and can be used to simplify the conversion process in calculations.
- 📝 When using conversion factors, it's important to use the factor that will cancel out the unit you're trying to convert from.
- 🔬 Understanding and applying the concepts of molar mass and significant figures are fundamental to accurately converting between grams and moles.
Q & A
What is the molar mass of aluminum and what does it signify?
-The molar mass of aluminum is twenty six point nine eight grams per mole (26.98 g/mol). It signifies the mass of one mole of aluminum atoms, which means that one mole of aluminum weighs 26.98 grams.
How do you convert moles of aluminum to grams using the molar mass?
-To convert moles of aluminum to grams, you multiply the number of moles by the molar mass of aluminum. For example, 4.30 moles of aluminum would be 4.30 moles multiplied by 26.98 g/mol, resulting in 116 grams of aluminum.
What is the significance of significant figures in the context of this problem?
-Significant figures are important because they indicate the precision of the measurement. In the context of this problem, the answer is rounded to three significant figures, which is consistent with the precision of the given data.
How can you use conversion factors to convert moles to grams?
-You can use conversion factors to convert moles to grams by setting up a ratio that equates one mole of a substance to its molar mass in grams. Then, you multiply the moles by this conversion factor to cancel out the moles and yield the mass in grams.
What is the molar mass of sodium chloride (NaCl) and how is it calculated?
-The molar mass of sodium chloride (NaCl) is fifty eight point four four grams per mole (58.44 g/mol). It is calculated by adding the molar masses of sodium (Na) and chlorine (Cl) from the periodic table, which are approximately 22.99 g/mol and 35.45 g/mol, respectively.
How do you convert grams of sodium chloride to moles?
-To convert grams of sodium chloride to moles, you divide the mass in grams by the molar mass of NaCl. For example, 127.5 grams of NaCl divided by 58.44 g/mol equals approximately 2.182 moles.
What is the relationship between the molar mass and the conversion of grams to moles?
-The molar mass is the mass of one mole of a substance. When converting grams to moles, you divide the total mass in grams by the molar mass to find out how many moles of the substance you have, since one mole of the substance weighs its molar mass in grams.
How does using conversion factors simplify the process of converting grams to moles?
-Using conversion factors simplifies the process by providing a direct relationship between the mass in grams and the number of moles. By multiplying the mass in grams by the conversion factor (which is the inverse of the molar mass), you can cancel out the units and obtain the number of moles directly.
What is the correct way to round the answer to a calculation involving significant figures?
-The correct way to round the answer is to retain the same number of significant figures as the original data with the least number of significant figures. You round the final result to the nearest significant figure based on the precision of the least precise value used in the calculation.
How does the process of converting moles to grams differ from converting grams to moles?
-Converting moles to grams involves multiplying the number of moles by the molar mass of the substance. Conversely, converting grams to moles involves dividing the mass of the substance in grams by its molar mass. The process differs in the mathematical operation used (multiplication versus division) but both rely on the molar mass for the conversion.
Why is it important to consider the molar mass when solving stoichiometry problems?
-The molar mass is important in stoichiometry because it provides the link between the amount of a substance (in moles) and its mass (in grams). It allows chemists to calculate the mass of reactants or products needed or produced in a chemical reaction, which is essential for understanding and conducting experiments in a lab.
What is the general rule for converting moles to grams and vice versa?
-The general rule for converting moles to grams is to multiply the number of moles by the molar mass of the substance. To convert grams to moles, divide the mass of the substance in grams by its molar mass.
Outlines
🔢 Converting Moles to Grams: The Molar Mass of Aluminum
This paragraph explains the concept of molar mass and how it's used to convert the number of moles of a substance into its mass in grams. The example given is for aluminum, with a molar mass of 26.98 grams per mole. The process involves multiplying the number of moles by the molar mass to find the total mass in grams. Additionally, the paragraph demonstrates how to use conversion factors to achieve the same result, emphasizing that the underlying math remains the same regardless of the method used.
🧮 Molar Mass Calculation: From Grams to Moles of Sodium Chloride
The second paragraph focuses on calculating the number of moles from a given mass. Using sodium chloride (NaCl) as an example, the molar mass is determined by adding the molar masses of sodium (22.99 g/mol) and chlorine (35.45 g/mol) to get a total of 58.44 g/mol for NaCl. The paragraph then describes how to find the number of moles by dividing the given mass (127.5 grams) by the molar mass. Both a direct calculation method and a conversion factor method are explained, showing that they both yield the same mathematical process and result of 2.182 moles when rounded to four significant figures.
🔄 Understanding Grams to Moles Conversion: A Summary
The final paragraph summarizes the process of converting between grams and moles, highlighting that regardless of the method used—whether it's a direct calculation or using conversion factors—the mathematical operations are the same. It emphasizes that to convert grams to moles, you divide the mass in grams by the molar mass of the substance. The paragraph concludes by encouraging viewers to seek additional practice problems for further understanding of the concept.
Mindmap
Keywords
💡Molar mass
💡Aluminum
💡Moles
💡Sodium chloride
💡Molar mass calculation
💡Conversion factors
💡Significant figures
💡Atomic mass
💡Periodic table
💡Chemical formula
💡Multiplication and division
Highlights
The video teaches how to convert between grams and moles, which is essential for understanding chemical quantities.
The molar mass of aluminum is provided as 26.98 grams per mole, a key piece of information for conversions.
A step-by-step multiplication method is demonstrated for finding the mass of multiple moles of a substance.
The concept of significant figures is introduced and its importance in scientific calculations is emphasized.
A simple rule for converting moles to grams by multiplying moles by the molar mass is presented.
Conversion factors are introduced as an alternative method for converting units in chemistry.
The process of using conversion factors to cancel out units and solve for the desired unit is explained.
The molar mass of sodium chloride (NaCl) is calculated by adding the molar masses of sodium and chlorine.
A method for converting grams to moles by dividing the mass of a substance by its molar mass is demonstrated.
The importance of rounding the final answer to the correct number of significant figures is discussed.
The video emphasizes that both the thinking-through method and the conversion factor method result in the same mathematical operations.
The video provides a clear distinction between the two methods, showcasing their equivalence in solving chemistry problems.
Practical applications of these conversions are highlighted, showing their relevance in chemistry and related fields.
The video concludes with an invitation to viewers to seek further practice in converting between grams and moles.
The transcript is a comprehensive guide for beginners to understand the basics of molar conversions in chemistry.
The video is structured to build from fundamental concepts to more complex problem-solving scenarios.
The use of the periodic table in determining molar masses is highlighted, underlining its importance as a chemist's tool.
The video employs both visual and verbal explanations to enhance understanding of the conversion process.
Transcripts
Browse More Related Video

Converting Between Grams and Moles (Part 2)

Molar Conversions: Grams to Moles and Moles to Grams
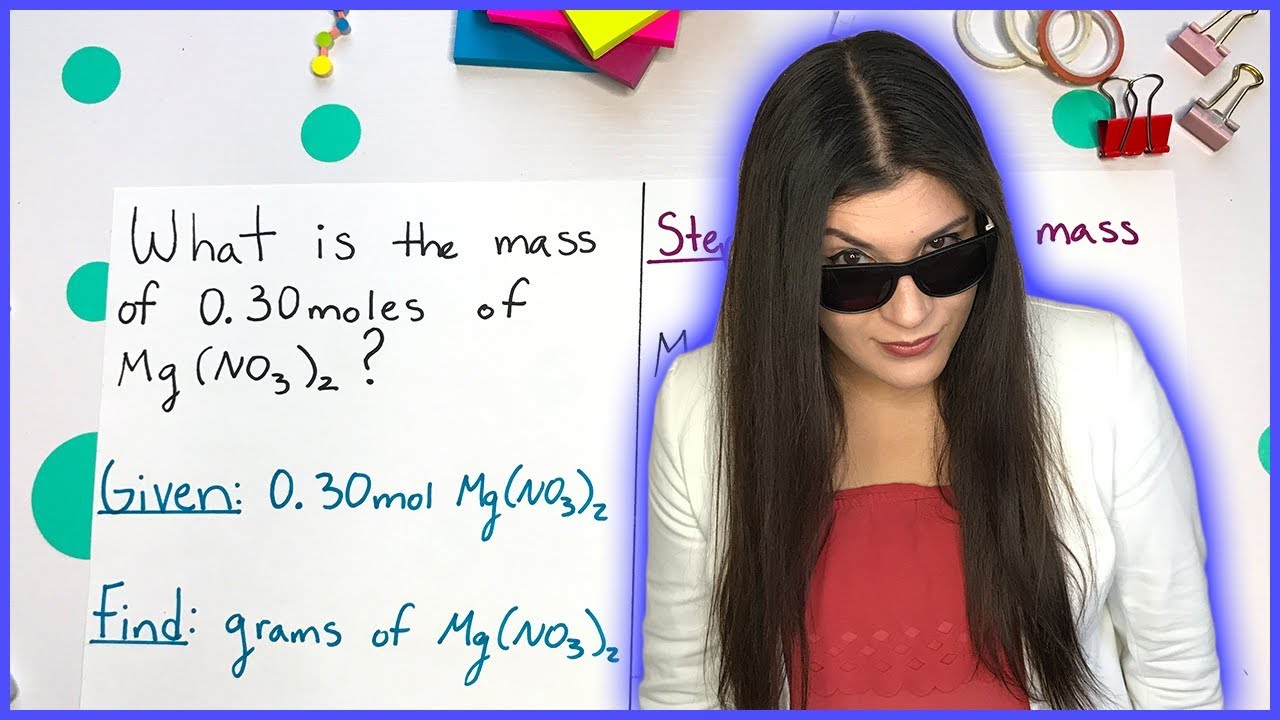
Converting Grams to Moles Using Molar Mass | How to Pass Chemistry
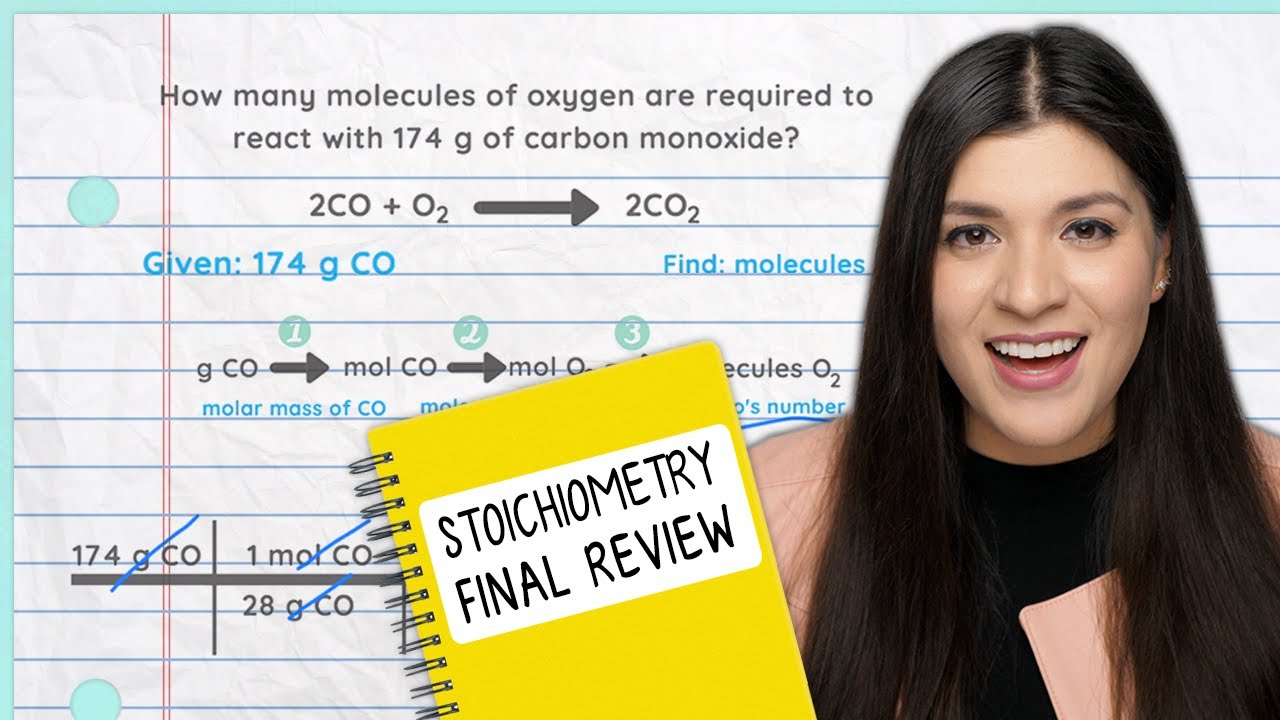
Know This For Your Chemistry Final Exam - Stoichiometry Review

Avogadro's Number, The Mole, Grams, Atoms, Molar Mass Calculations - Introduction
Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: