Ranking Acid Base Strength Using Ka pKa Values Leah4sci
TLDRThis video delves into the concepts of acid and base strength through the lens of Ka and pKa values, emphasizing their inverse relationship and its implications for understanding the dissociation of acids in solution. It introduces a mnemonic, 'CARRY O', to remember factors influencing the stability of conjugate bases, which is key to predicting the strength of an acid. The video also encourages a relatable approach to molecules, comparing their behavior to human emotions, and offers resources for further study and practice in organic chemistry.
Takeaways
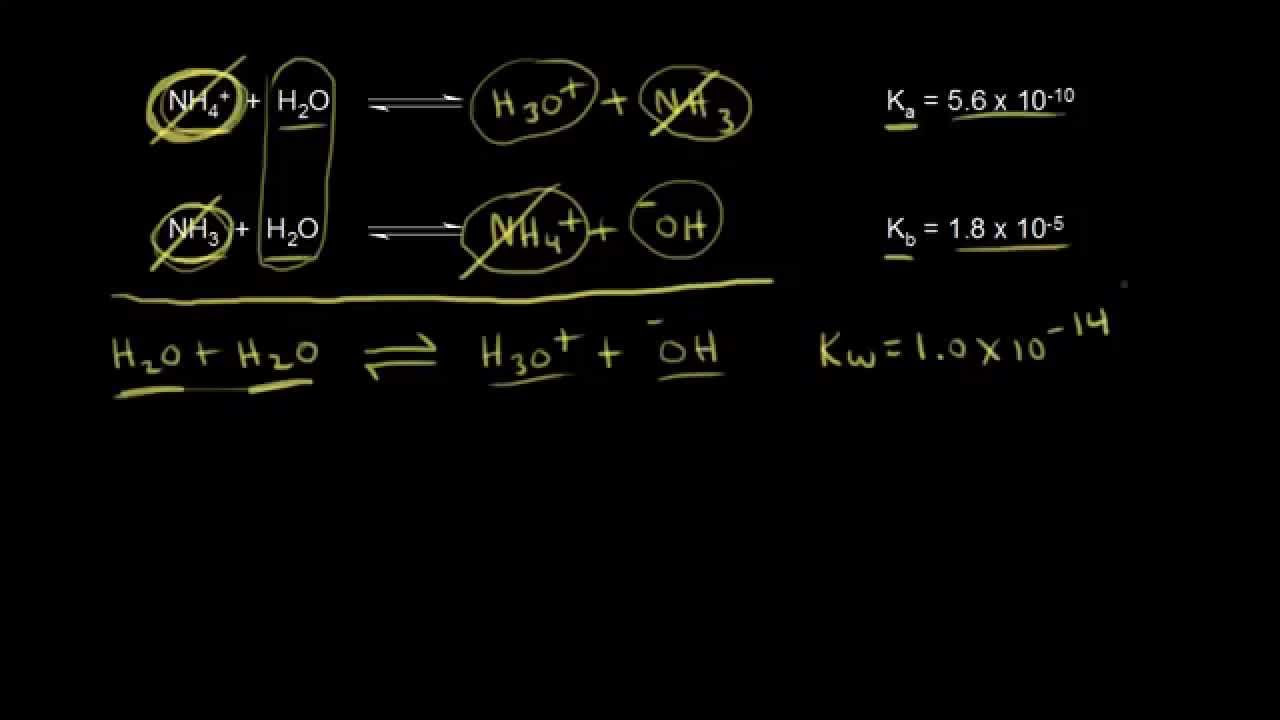
- 📚 Understanding the strength of an acid or base is done using Ka or pKa values, which are essential for ranking their relative strengths without performing calculations.
- 🔢 Ka is the acid dissociation constant and is directly proportional to the H+ concentration; a high Ka indicates a strong acid, while a low Ka indicates a weak acid.
- 📈 pKa is the negative logarithm of Ka, meaning that a high Ka corresponds to a low pKa and vice versa, making them inversely proportional.
- 🧪 In organic chemistry, the focus is on understanding the concepts to rank acids and bases rather than performing lengthy calculations.
- 📊 To compare the acidity of molecules not provided with Ka or pKa values, one must use trends and the acid-base equation to determine stability and strength.
- 🌟 The stability of a conjugate base (A-) is crucial in determining the strength of an acid; a stable A- indicates a strong acid, while an unstable A- indicates a weak acid.
- 💡 A strong acid is one that readily gives up its H+ and remains stable as a conjugate base, leading to a higher concentration of H+ in solution.
- 🚫 A weak acid is one where the conjugate base (A-) is unstable and reactive, causing it to reassociate with H+ instead of staying dissociated.
- 📚 The script suggests using human characteristics to understand molecules, where happy and stable molecules are less reactive, and unhappy and unstable molecules are more reactive.
- 🎓 The video series and additional resources like the practice quiz and cheat sheet can be found on the website layorsicom.com/acid-base, and an ebook '10 Secrets to Acing Organic Chemistry' is available for free at orosecrets.com.
Q & A
What is the main topic of the video?
-The main topic of the video is understanding the strength of acids and bases using Ka or pKa values and logical reasoning in the context of organic chemistry.
What is the acid dissociation constant (Ka) and how is it related to the concentration of H+ ions?
-The acid dissociation constant (Ka) is a measure of the strength of an acid. It is equal to the concentration of H+ ions (proton concentration) in a solution. A higher Ka value indicates a higher H+ concentration, which means a stronger acid, while a lower Ka value indicates a weaker acid.
What is the relationship between Ka and pKa values?
-pKa is the negative logarithm of the Ka value. This means that as Ka increases (indicating a stronger acid), pKa decreases, and vice versa. So, a high Ka corresponds to a low pKa, and a low Ka corresponds to a high pKa, with the former indicating a strong acid and the latter indicating a weak acid.
How can you determine the strength of an acid or base without Ka or pKa values?
-Without Ka or pKa values, you can determine the strength of an acid or base by examining the molecular structure, writing out the acid-base equation, comparing the acids and their conjugate bases, and using a list of trends to assess which is more stable. A stable molecule is less likely to react and will form a stronger acid, while an unstable molecule is more reactive and will form a weaker acid.
What are the 'five factors' mentioned in the video that help in understanding the stability of a conjugate base?
-The 'five factors' referred to in the video are: charged atom, holding the charge, resonance, inductive effect, and orbitals. These factors will be discussed in more detail in upcoming videos and are crucial for understanding the stability and reactivity of conjugate bases in acid-base reactions.
What is the 'golden rule' for understanding organic or any scientific reaction as mentioned in the video?
-The 'golden rule' mentioned in the video is 'happy stable, unreactive; unhappy, unstable, reactive.' This rule suggests that molecules that are stable and 'happy' are less likely to react, while those that are unstable and 'unhappy' are more likely to react.
How does the video relate human emotions to the behavior of acids and bases?
-The video uses a metaphor where stable and happy molecules are compared to people in a good mood who are less likely to react aggressively, while unstable and unhappy molecules are compared to people in a bad mood who are more likely to react and cause problems. This analogy helps to understand that a strong acid is one that readily gives up its proton (happy and stable as a conjugate base), while a weak acid is one that does not want to give up its proton (unhappy and unstable as a conjugate base).
What is the significance of the conjugate base (A-) in determining the strength of an acid?
-The conjugate base (A-) plays a crucial role in determining the strength of an acid. If the conjugate base is stable and 'happy,' it will not readily react to regain the proton, leading to a continuous dissociation of the acid and a higher concentration of H+ ions, making it a stronger acid. Conversely, if the conjugate base is unstable and 'unhappy,' it will react to regain the proton, leading to less H+ ions in solution and making it a weaker acid.
How can you increase your understanding and success in organic chemistry according to the video?
-The video suggests visiting the speaker's website for resources such as practice quizzes, cheat sheets, and an ebook titled '10 Secrets to Acing Organic Chemistry.' These resources, along with the speaker's video series, can help guide students through the course and improve their understanding and success in organic chemistry.
What are the practical applications of understanding acid and base strength?
-Understanding acid and base strength is fundamental in organic chemistry and has practical applications in various fields such as pharmaceuticals, chemical engineering, environmental science, and even in everyday life. It helps in predicting reactions, designing chemical processes, and understanding natural phenomena.
What is the role of the inductive effect in the stability of a conjugate base?
-The inductive effect plays a significant role in the stability of a conjugate base. It refers to the influence that the surrounding atoms in a molecule have on the reactivity and stability of a particular atom or group. In the context of conjugate bases, the inductive effect can either stabilize or destabilize the negative charge, affecting the overall reactivity and strength of the acid.
Outlines
📚 Understanding Acids and Bases with Ka and pKa
This paragraph introduces the concept of using Ka and pKa values to understand the strength of an acid or base. It emphasizes the importance of not just memorizing values but understanding the underlying logic. The Ka value is directly proportional to the H+ concentration, indicating that a higher Ka corresponds to a stronger acid, while a lower Ka indicates a weaker acid. The relationship between Ka and pKa is inversely proportional, meaning a high Ka results in a low pKa and vice versa. The paragraph also suggests using logic and human characteristics to predict the behavior of molecules in acid-base reactions.
🧪 Humanizing Molecules: Stability and Reactivity
The second paragraph uses a relatable analogy to explain the behavior of acids and bases in terms of stability and reactivity. It compares the release of a proton (H+) from an acid to a person's mood and how it reacts to situations. A stable, happy molecule (a-) does not want to attack other molecules or regain the proton, leading to a stronger acid. In contrast, an unstable, unhappy a- is reactive and wants to re-form the original acid, resulting in a weaker acid. The paragraph also introduces the 'carry o' mnemonic, which stands for charged atom, holding the charge, resonance inductive effect, and orbitals, as factors to analyze the stability of a conjugate base.
📈 Resources and Support for Organic Chemistry Students
The final paragraph focuses on providing resources for students struggling with organic chemistry. It encourages viewers to subscribe to the channel for more content and offers a free ebook titled '10 Secrets to Acing Organic Chemistry' for those looking to excel in the subject. The ebook and additional study materials, such as cheat sheets and reaction guides, can be accessed through a provided link, along with exclusive email updates and notifications of new videos.
Mindmap
Keywords
💡Acid Dissociation Constant (Ka)
💡pKa
💡Conjugate Base
💡Hydrogen Ion (H+)
💡Organic Chemistry
💡Carry O
💡Stability
💡Reactivity
💡Human Characteristics
💡Acid-Base Equation
💡Study Tips
Highlights
Understanding the strength of an acid or base using Ka or pKa values is discussed in the video.
The relationship between Ka and H+ concentration is directly proportional, meaning a high Ka value indicates a strong acid.
pKa is the negative log of Ka, which inversely relates to the strength of an acid, with a low Ka value corresponding to a high pKa value.
In organic chemistry, the focus is on ranking acids and bases conceptually rather than performing lengthy calculations.
The video emphasizes the importance of understanding the logic behind acid-base concepts rather than just memorizing values.
Ranking molecules based on their acid-base properties involves writing out the equation and comparing the conjugate bases.
The video introduces a mnemonic, CARRY O, for factors to analyze the stability of a conjugate base: Charged Atom, Resonance, Inductive Effect, and Orbitals.
A strong acid is one that will happily give up its H+ because its conjugate base is stable and unreactive.
A weak acid is unstable and wants to reassociate, resulting in a low concentration of H+ in solution.
The video uses a relatable analogy of human emotions to explain the behavior of acids and bases in different situations.
The concept of 'happy stable, unreactive' and 'unhappy unstable, reactive' is introduced to help understand the behavior of acids and bases.
The video provides a link to additional resources, including a practice quiz and cheat sheet, for those interested in further learning about acid-base concepts.
The video offers a free ebook, '10 Secrets to Acing Organic Chemistry', for viewers to enhance their understanding and performance in the subject.
The video encourages viewers to subscribe to the channel for ongoing content related to organic chemistry.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: