19. Quantum Mechanics I: The key experiments and wave-particle duality
TLDRIn this engaging lecture, the professor introduces the paradoxical world of quantum mechanics, emphasizing its counterintuitive nature. The session focuses on the dual-particle wave concept, exemplified by the famous double-slit experiment. It explains how electrons and photons exhibit both wave and particle characteristics, with their behavior influenced by observation. The lecture also delves into the uncertainty principle, highlighting the inherent trade-off between the precision of position and momentum measurements. The complex exponential function is introduced as the wave function for a particle with definite momentum, a fundamental concept in quantum mechanics.
Takeaways
- 🌟 Quantum mechanics is a complex subject that challenges our intuitive understanding of the physical world, as even renowned physicists like Richard Feynman acknowledged its perplexing nature.
- 📚 The professor aims to transition the students from understanding quantum mechanics to being able to teach and spread the knowledge, emphasizing the importance of sharing scientific insights.
- 🚀 The course will be an adventure, encouraging students to think beyond exams and grades, and to appreciate the historical significance and marvel of quantum mechanics as a major scientific discovery.
- 🧠 The human understanding of quantum mechanics is not absolute and is subject to change with new experiments and refined measurements, highlighting the iterative and evolving nature of scientific theories.
- 🌀 The double-slit experiment is a pivotal demonstration of the wave-particle duality, showing that light exhibits characteristics of both waves and particles depending on the conditions of the experiment.
- 💡 The superposition principle is crucial in understanding the interference pattern observed in the double-slit experiment, where the electric field - not the intensity - is added when two slits are open.
- 📈 The photoelectric effect and Compton scattering are key experiments that provided evidence for the particle nature of light, with photons carrying discrete packets of energy and momentum.
- 🌊 The wave aspect of particles like electrons and photons is described by a wave function, which encodes the probability of finding the particle at a certain location and is linked to the particle's momentum.
- 🔬 The uncertainty principle arises from the wave nature of particles, stating that the more precisely the position (ℏ) is known, the less precisely the momentum can be determined, and vice versa.
- 👁️ Observation and measurement in quantum mechanics have profound effects on particles, as the act of measuring a particle's position inherently alters its momentum due to the interaction with the photons used for observation.
Q & A
What is the main challenge in understanding quantum mechanics?
-The main challenge in understanding quantum mechanics is its counterintuitive nature, as it involves concepts that are difficult to grasp and visualize, such as particles exhibiting both wave-like and particle-like behaviors.
What did Richard Feynman famously say about quantum mechanics?
-Richard Feynman famously said, "No one understands quantum mechanics," highlighting the difficulty and complexity of the subject.
What is the significance of the double-slit experiment in quantum mechanics?
-The double-slit experiment is significant in quantum mechanics because it demonstrates the wave-particle duality of light and other particles, showing that they can exhibit both wave-like interference patterns and particle-like behavior.
What is the superposition principle in quantum mechanics?
-The superposition principle in quantum mechanics states that a quantum system can exist in multiple states simultaneously until it is measured, at which point it collapses into a single state.
What is the photoelectric effect and how does it relate to photons?
-The photoelectric effect is the emission of electrons from a material when it is exposed to light of a certain frequency. It relates to photons because it provides evidence for the particle nature of light, with each photon carrying a quantized amount of energy that can eject an electron from a material.
What is the connection between a photon's energy and momentum?
-The connection between a photon's energy (E) and momentum (p) is given by the relation E = pc, where c is the speed of light. This relation is derived from the fact that the energy of a photon is proportional to its frequency (E = ħω) and its momentum is proportional to its wave number (p = ħk).
What is the de Broglie wavelength associated with a particle of momentum p?
-The de Broglie wavelength associated with a particle of momentum p is given by the formula λ = h/p, where h is Planck's constant and p is the momentum of the particle.
How does the act of observation affect the behavior of quantum particles?
-The act of observation in quantum mechanics can change the behavior of particles. For example, attempting to measure the position of an electron with high precision can disturb its momentum due to the interaction with the photons used for observation, leading to an uncertainty in its momentum.
What is the uncertainty principle and what does it imply?
-The uncertainty principle, introduced by Werner Heisenberg, states that there is a fundamental limit to the precision with which certain pairs of physical properties, such as position (x) and momentum (p), can be known simultaneously. It implies that the more precisely one property is measured, the less precisely the other can be known, and vice versa.
What is the role of wave functions in quantum mechanics?
-Wave functions in quantum mechanics provide a mathematical description of the probability distribution of a particle's presence in space. The square of the wave function (|ψ|^2) at a particular point gives the probability density of finding the particle at that location.
How does quantum mechanics differ from classical mechanics in terms of particle description?
-In classical mechanics, particles are described with definite positions and momenta. In contrast, quantum mechanics introduces the concept of wave-particle duality, where particles are described by wave functions that provide information about the probabilities of finding the particle in various locations, rather than exact positions and momenta.
Outlines
📚 Introduction to Quantum Mechanics
The professor introduces the topic of quantum mechanics, acknowledging its complexity and the counter-intuitive nature of the subject. He references Richard Feynman's famous quote about the difficulty of understanding quantum mechanics, emphasizing that it's normal for students to struggle with the concepts. The goal is to embrace the challenge and view the learning process as an adventure, focusing on the significance of quantum mechanics in the realm of physics and science as a whole.
🌀 The Double Slit Experiment and Wave-Particle Duality
The discussion delves into the famous double slit experiment, highlighting the phenomenon of interference when both slits are open. The professor explains the superposition principle and how it relates to the wave function, contrasting it with classical expectations of particle behavior. The experiment demonstrates that light exhibits both wave-like and particle-like properties, challenging traditional views of physics and leading to the concept of wave-particle duality.
🤔 The Puzzle of Light as Particles
The professor explores the unexpected behavior of light when it is observed as particles, specifically when individual photons interact with a detector. The surprising finding is that light, traditionally considered a wave, behaves as if it is made up of discrete particles, or photons, each carrying a specific energy and momentum. This realization is further supported by the photoelectric effect and Compton scattering, reinforcing the dual nature of light.
🌟 The Photoelectric Effect and Compton Scattering
The lecture continues with a deeper examination of the photoelectric effect and Compton scattering, both of which provide strong evidence for the particle nature of light. The photoelectric effect demonstrates that photons carry discrete packets of energy, while Compton scattering shows that photons also possess momentum. These phenomena, along with the double slit experiment, confirm the wave-particle duality and the existence of photons as massless particles.
🤯 The Impact of Observation on Quantum Systems
The professor discusses the critical role of observation in quantum mechanics, particularly how the act of measuring can influence the behavior of particles. The example of electrons passing through a double slit and being detected illustrates how observation can collapse the wave function, leading to different outcomes. This raises profound questions about the nature of reality and the fundamental principles of quantum mechanics.
🌈 The Wave Function and Uncertainty Principle
The lecture concludes with an exploration of the wave function and the uncertainty principle. The professor explains that the wave function, represented by a complex exponential for a particle with definite momentum, provides the probabilities of finding a particle in a particular location. The uncertainty principle emerges as a fundamental concept, stating that the more precisely the position of a particle is known, the less precisely its momentum can be determined, and vice versa.
Mindmap
Keywords
💡Quantum Mechanics
💡Wave-Particle Duality
💡Double-Slit Experiment
💡Richard Feynman
💡Uncertainty Principle
💡Interference
💡Photoelectric Effect
💡Compton Scattering
💡Wave Function
💡Planck's Constant
Highlights
The introduction of quantum mechanics and its inherent complexity due to its non-intuitive nature is discussed, with a focus on the famous quote by Richard Feynman that "No one understands quantum mechanics."
The professor's goal to make students unable to understand quantum mechanics, not just in the short term but as a tool to spread the understanding of its complexity everywhere.
Quantum mechanics is presented as an adventure, encouraging students to think beyond exams and grades, and to appreciate it as one of the biggest discoveries in physics.
The historical approach to teaching quantum mechanics is critiqued, with the suggestion that it can lead to confusion due to the many wrong turns and battles in the development of the theory.
The double-slit experiment is introduced as a starting point to understand the issues with Newtonian mechanics and Maxwell's theory.
The phenomenon of interference is explained, highlighting the superposition principle and the difference between adding electric fields and intensities.
The transition from viewing light as a wave to understanding it as made up of discrete particles, or photons, is discussed, challenging traditional notions of light as a continuous wave.
The photoelectric effect is explained as indirect evidence for the existence of photons, with Einstein's work on this phenomenon earning him the Nobel Prize.
Compton scattering is introduced as another experiment that confirms the existence of photons, showing that light can be characterized by both wavelength and momentum, as well as frequency and energy.
The wave-particle duality of light is explored, with the understanding that light exhibits both wave-like and particle-like properties depending on the experimental conditions.
de Broglie's hypothesis is presented, suggesting that if light has a wave-particle duality, then particles like electrons should also exhibit wave-like behavior.
The importance of observation in quantum mechanics is highlighted, showing that the act of observing a quantum system can change its behavior, as demonstrated by the change in the interference pattern when electrons are observed going through one slit or the other.
The uncertainty principle is introduced, stating that it is impossible to precisely know both the position and momentum of a particle at the same time, with the product of the uncertainties being of the order of ħ.
The wave function is introduced as a mathematical construct used to calculate the probability of finding a particle in a particular state, with its square representing the probability density.
The complex exponential function is identified as the form of the wave function for a particle with definite momentum, satisfying both the wavelength requirement and the uncertainty principle.
The limitations of classical mechanics in describing quantum phenomena are emphasized, with quantum mechanics providing a more accurate framework for understanding the behavior of particles at the microscopic scale.
Transcripts
Browse More Related Video
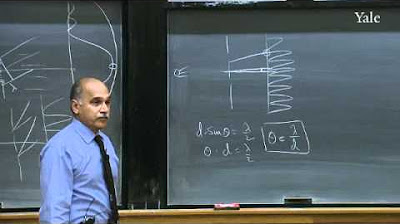
20. Quantum Mechanics II
21. Quantum Mechanics III
What Is Quantum Mechanics Explained

Lec-11 I Introduction to quantum chemistry I Applied Chemistry I Chemical Engineering
Are Photons & Electrons Particles or Waves? Make up your mind god!

22. Quantum mechanics IV: Measurement theory, states of definite energy
5.0 / 5 (0 votes)
Thanks for rating: