What Is Quantum Mechanics Explained
TLDRThis script explores the elegance and significance of the Schrödinger wave equation in quantum mechanics, contrasting the simplicity of its formulation with the complex behaviors it explains. It delves into the atomic and subatomic world, where classical physics falls short, and introduces the concept of wave-particle duality, illustrated by the famous double-slit experiment. The script also touches on the probabilistic nature of quantum mechanics, the uncertainty principle, and the phenomenon of entanglement, highlighting the counterintuitive yet fundamental aspects of the quantum realm.
Takeaways
- 📚 The Schrödinger wave equation is a fundamental and elegant equation in quantum mechanics, reflecting the simplicity often found in important physical theories.
- 🌌 Quantum mechanics is the study of atomic and subatomic particles, which are the building blocks of the macroscopic world we experience daily.
- 🔬 Classical physics, like Newton's laws, is well-suited for macroscopic objects but fails to accurately describe the behavior of particles at the atomic and subatomic levels.
- 🌐 The wave-particle duality is a central concept in quantum mechanics, where particles like electrons exhibit both wave-like and particle-like properties.
- 💡 The double-slit experiment is a key demonstration of wave-particle duality, showing that particles can create interference patterns typically associated with waves.
- 🧬 Quantum mechanics is probabilistic, meaning that it describes the likelihood of different outcomes rather than predicting specific results with certainty.
- 🔍 The Heisenberg uncertainty principle states that it is impossible to simultaneously know the exact position and momentum of a particle.
- 🔗 Quantum entanglement is a phenomenon where the state of one particle is instantly correlated with the state of another, no matter the distance between them.
- 📊 The behavior of particles in quantum mechanics is described by wave functions, which are mathematical representations that evolve according to the Schrödinger equation.
- 🚫 Quantum mechanics challenges our intuition, as it cannot be fully understood through everyday experiences and requires a different conceptual framework.
- 🔄 Quantum mechanics successfully bridges the gap between the microscopic world and the macroscopic world, providing a framework that approximates classical physics for larger systems.
Q & A
What is the Schrödinger wave equation and why is it significant?
-The Schrödinger wave equation is a fundamental equation in quantum mechanics that describes how the quantum state of a physical system changes over time. It is significant because it is simple and elegant, and it serves as the starting point for understanding quantum mechanics.
What does Einstein's quote about the simplicity of a good theory imply?
-Einstein's quote implies that the most effective theories are those that can explain phenomena with the fewest and simplest elements. This is because simplicity often leads to a more profound understanding of the underlying principles of nature.
How does the script relate the expression of emotions to the simplicity of physical theories?
-The script compares the expression of emotions to the simplicity of physical theories by suggesting that, just as we can express our thoughts more efficiently and concisely when written down, so too can physical theories be more powerful when they are simple and elegant.
What is the main difference between the macroscopic and atomic/subatomic worlds?
-The main difference is that the macroscopic world, which includes objects we interact with daily, is well described by classical physics, while the atomic and subatomic world requires quantum mechanics for accurate description due to phenomena like wave-particle duality.
Why did classical physics fail to describe the atomic and subatomic world?
-Classical physics failed because it could not account for the observed phenomena at the atomic and subatomic levels, such as the dual wave-particle nature of matter, which necessitated the development of quantum mechanics.
What is wave-particle duality and why is it important in quantum mechanics?
-Wave-particle duality is the concept that every particle or quantum entity can exhibit both wave and particle properties. It is important because it challenges our classical understanding of matter and is a fundamental aspect of quantum mechanics.
What is the double-slit experiment and what does it demonstrate?
-The double-slit experiment is a demonstration of the wave-particle duality of light and matter. It shows that particles like electrons can create an interference pattern on a screen, similar to waves, indicating their wave-like behavior.
What is a wave function in quantum mechanics and what role does it play?
-A wave function, represented by the Greek letter 'psi' (ψ), is a mathematical description of the quantum state of a particle or system. It plays a crucial role in quantum mechanics as it contains all the information needed to predict the behavior and properties of the system.
How does the concept of the uncertainty principle relate to quantum mechanics?
-The uncertainty principle states that it is impossible to simultaneously know both the exact position and momentum of a particle. This principle is a fundamental concept in quantum mechanics, highlighting the probabilistic nature of the quantum world.
What is entanglement in quantum mechanics and why is it significant?
-Entanglement is a phenomenon where two or more particles become linked and the state of one instantaneously influences the state of the other, regardless of the distance between them. It is significant because it challenges our classical understanding of causality and highlights the non-local properties of quantum systems.
How does quantum mechanics describe the macroscopic world?
-Quantum mechanics describes the macroscopic world by approximating the classical description of nature in the case of large systems. It means that in the limit of a large number of particles, quantum mechanics reproduces the deterministic behavior observed in the macroscopic world.
What did Richard Feynman mean when he said quantum physics deals with 'nature as she is – absurd'?
-Feynman meant that quantum physics reveals a nature that is fundamentally different from our everyday experiences and intuitions, often exhibiting behaviors that seem absurd or counterintuitive, such as the probabilistic nature of events and the impossibility of knowing certain properties simultaneously.
Outlines
📚 The Beauty and Significance of Schrödinger's Equation
The paragraph introduces the Schrödinger wave equation as one of the most crucial equations in physics due to its simplicity and elegance, which are traits often found in fundamental formulas. It likens a well-constructed theory to a concise expression of emotions, emphasizing the importance of simplicity in both. The paragraph also sets the stage for quantum mechanics by explaining that it deals with atomic and subatomic particles, contrasting it with classical physics which governs the macroscopic world. The inadequacy of classical physics at the atomic level led to the development of quantum mechanics, highlighting the wave-particle duality of light and matter as a central concept.
🌌 The Quantum World: Wave-Particle Duality and Observation Effects
This section delves into the double-slit experiment, which illustrates the wave-like behavior of particles such as electrons. It explains how the act of observation affects the outcome, a key quantum phenomenon. The paragraph contrasts the expected result of particle behavior with the actual wave interference pattern observed, emphasizing the probabilistic nature of quantum mechanics. It introduces the wave function, represented by the Greek letter 'psi', and its role in Schrödinger's equation, which describes the evolution of a system's state. The summary also touches on the counterintuitive aspects of quantum mechanics, such as the inability to predict exact outcomes and the probabilistic nature of reality at the quantum level.
🔬 Quantum Mechanics: Probabilistic Foundations and Macroscopic Implications
The final paragraph discusses the probabilistic nature of quantum mechanics, contrasting it with the deterministic view of the world that prevailed before. It explains the uncertainty principle, which limits the precision with which certain pairs of physical properties can be known simultaneously. The paragraph also covers the concept of entanglement, where the state of one particle is intrinsically linked to another, regardless of distance. It concludes by affirming the validity of quantum mechanics, which, despite its focus on the very small, successfully approximates the classical description of nature for larger systems. The summary ends with a quote from Richard Feynman, highlighting the absurdity of quantum physics, and an invitation for viewers to engage with the content.
Mindmap
Keywords
💡Schrödinger wave equation
💡Quantum mechanics
💡Wave-particle duality
💡Double-slit experiment
💡Wave function
💡Uncertainty principle
💡Entanglement
💡Classical physics
💡Probability
💡Complementarity
💡Measurement
Highlights
Schrödinger's wave equation is introduced as the most important and beautiful equation in quantum mechanics due to its simplicity and elegance.
Einstein's belief in the simplicity of the universe and the importance of elegant formulas in physics is discussed.
The comparison between expressing emotions verbally and writing them down is used to illustrate the value of concise theories in physics.
Quantum mechanics is defined as the study of atomic and subatomic particles, contrasting with the macroscopic world described by classical physics.
The inadequacy of classical physics in explaining the atomic and subatomic world led to the development of quantum mechanics.
Light's dual nature of behaving as both particles and waves is highlighted as a key aspect of quantum mechanics.
Wave-particle duality is a fundamental concept of quantum mechanics, applicable to elementary and compound particles.
Einstein's perspective on the difficulty of reconciling the particle and wave theories of light is presented.
The double-slit experiment is described as evidence of the dual nature of matter, showing particles can exhibit wave-like behavior.
The concept of a wave function, represented by the Greek letter 'psi', is introduced as central to quantum mechanics.
Schrödinger's equation is explained as determining the temporal evolution of a system's state in quantum mechanics.
Quantum mechanics is distinguished from classical physics by its reliance on probability rather than deterministic outcomes.
The Heisenberg uncertainty principle is introduced, stating the impossibility of precisely knowing both position and speed of a particle simultaneously.
Quantum entanglement is mentioned as an example of the non-intuitive phenomena in quantum mechanics where measurements on one particle affect another, regardless of distance.
Quantum mechanics is shown to be a probabilistic description of nature, contrasting with the deterministic view of classical physics.
The importance of quantum mechanics in approximating classical descriptions for large systems is emphasized.
Feynman's quote about quantum physics dealing with 'nature as she is – absurd' is used to highlight the counterintuitive aspects of quantum mechanics.
The video concludes with an invitation for viewers to share their thoughts on quantum mechanics in the comments.
Transcripts
Browse More Related Video
19. Quantum Mechanics I: The key experiments and wave-particle duality

The SIMPLEST Explanation of QUANTUM MECHANICS in the Universe!
20. Quantum Mechanics II

Lecture 7 | Quantum Entanglements, Part 1 (Stanford)

The Trouble with Gravity: Why Can't Quantum Mechanics explain it?
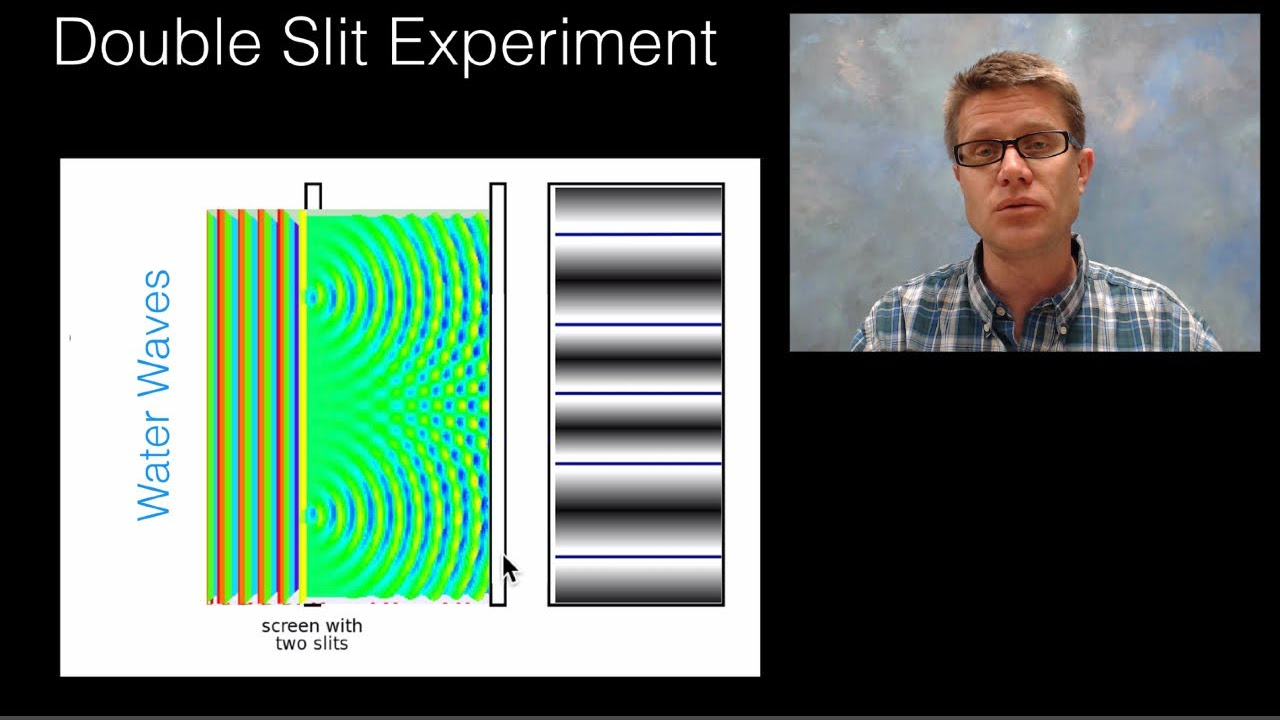
Wave-Particle Duality - Part 1
5.0 / 5 (0 votes)
Thanks for rating: