Reactions in equilibrium | Chemical equilibrium | Chemistry | Khan Academy
TLDRThis transcript delves into the concept of chemical equilibrium, contrasting one-way reactions with reversible ones. It explains the energy dynamics using an energy diagram and introduces the equilibrium constant, detailing its calculation and significance in predicting reaction direction and concentration changes at equilibrium. The example of the Haber process is used to illustrate these concepts, highlighting the balance between forward and reverse reactions and the implications of the equilibrium constant's value on the favored direction of reaction.
Takeaways
- 🧪化学反应可以是单向的,也可以是双向的,双向反应达到平衡时,正逆反应速率相等。
- 📈能量图解可以帮助理解反应的进程,从高能量状态到低能量状态,可能存在一个激活能的障碍。
- ⚖️化学平衡研究的是不同分子的浓度,当正逆反应速率相等时,系统达到平衡状态。
- 🔄达到化学平衡并不意味着各种物质的浓度相等,而是它们的浓度不再随时间变化。
- 🌡️化学平衡常数(K)是在特定温度下定义的,它反映了平衡时产物与反应物浓度的比值。
- 📊平衡常数的表达式是产物浓度的乘积(按它们的系数次幂)除以反应物浓度的乘积(同样按系数次幂)。
- 📚在介绍化学动力学时,讨论的是反应发生的速度以及如何改变活化能来影响这个速度。
- 🔄平衡反应可以用双向箭头表示,显示物质可以同时向两个方向转化。
- 🌟哈柏过程(Haber process)是一个实际的化学平衡例子,描述了氮气和氢气生成氨的过程。
- 🔢通过已知的平衡常数和一些物质的浓度,可以计算出其他物质在平衡时的浓度。
- 💡平衡常数小于1表示逆反应更受青睐,而大于1则表示正反应更受青睐。
Q & A
What is the key concept discussed in the script?
-The key concept discussed in the script is chemical equilibrium, which refers to a state in a chemical reaction where the rate of the forward reaction equals the rate of the reverse reaction, leading to a stable concentration of products and reactants.
How is equilibrium different from kinetics?
-Equilibrium focuses on the concentrations of products and reactants once the rates of the forward and reverse reactions become equal, whereas kinetics examines the speed at which reactions occur and factors influencing reaction rates.
What does the equilibrium constant represent?
-The equilibrium constant represents the ratio of the concentrations of products to reactants at equilibrium, raised to the power of their respective stoichiometric coefficients.
How is the equilibrium constant calculated?
-The equilibrium constant (Kc) is calculated by multiplying the concentrations of products, each raised to the power of their stoichiometric coefficients, and dividing by the concentrations of reactants, also raised to the power of their respective coefficients.
What does an equilibrium constant less than 1 indicate about the reaction?
-An equilibrium constant less than 1 indicates that the concentration of reactants is greater than the concentration of products at equilibrium, suggesting that the reverse reaction is favored.
How does changing the concentration of reactants affect the equilibrium constant?
-Changing the concentration of reactants does not affect the equilibrium constant, as it is a constant value determined by the reaction's stoichiometry and temperature.
What is the significance of temperature in relation to the equilibrium constant?
-The equilibrium constant is only valid for a given temperature. Changes in temperature can alter the equilibrium constant value, as it influences reaction rates and shifts the equilibrium position.
What is the intuition behind defining the equilibrium constant in terms of concentrations?
-Defining the equilibrium constant in terms of concentrations provides a standardized measure independent of reaction scale, allowing for predictions of equilibrium concentrations regardless of initial reactant amounts.
Why is the equilibrium constant useful in predicting reaction outcomes?
-The equilibrium constant allows for the prediction of equilibrium concentrations based on initial reactant concentrations, facilitating understanding of reaction directionality and the relative amounts of products and reactants at equilibrium.
What is the Haber process, and how does it relate to equilibrium?
-The Haber process involves the synthesis of ammonia from nitrogen and hydrogen gases and occurs at equilibrium. It serves as an example to illustrate the principles of equilibrium and the calculation of equilibrium constants.
Outlines
🌟 Understanding Chemical Reactions and Equilibrium
This paragraph introduces the concept of chemical reactions, focusing on the transformation of reactants to products. It explains the one-way reaction model, where reactants such as molecule A and B transform into products C and D. The energy diagram is used to illustrate the reaction's progression from a higher energy state to a lower, more stable energy state. The paragraph also introduces the idea of reactions that can proceed in both directions, leading to a state of equilibrium where the rate of the forward reaction equals the rate of the reverse reaction. The concept of activation energy is briefly touched upon, and the Haber process is mentioned as an example of an equilibrium reaction.
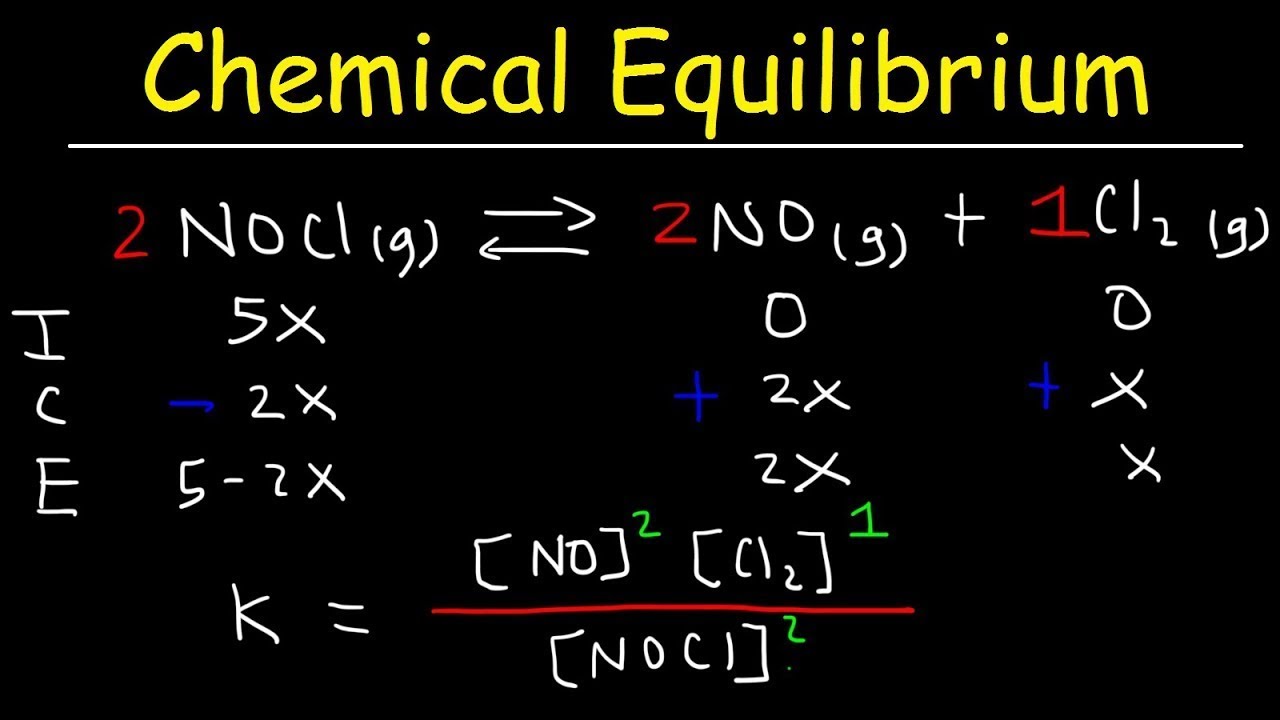
📈 The Equilibrium Constant and Its Significance
This paragraph delves into the specifics of equilibrium reactions, highlighting the equilibrium constant as a key factor. It explains how the equilibrium constant is calculated by taking the concentration of the products raised to their respective mole ratios and dividing by the concentration of the reactants. The paragraph emphasizes that the equilibrium constant is temperature-dependent and provides an example using the Haber process to illustrate the calculation. It also discusses the implications of the equilibrium constant's value, indicating whether the reaction favors the forward or reverse direction.
🔄 Exploring the Dynamics of Equilibrium Constants
The final paragraph further explores the equilibrium constant, discussing its role in predicting reaction outcomes. It explains how the equilibrium constant, once established for a specific temperature, allows for the prediction of resulting concentrations despite changes in reactant concentrations. The paragraph uses a hypothetical example to demonstrate the calculation of the equilibrium constant and its interpretation. It also touches on the implications of adding more reactants to the reaction and how this affects the equilibrium constant, promising further discussion in the next video on the subject.
Mindmap
Keywords
💡Reaction
💡Activation Energy
💡Equilibrium
💡Equilibrium Constant
💡Concentration
💡Molarity
💡Stoichiometry
💡Kinetics
💡Energy Diagram
💡Stability
💡Reactants
💡Products
Highlights
Chemical reactions can occur in both one direction and in both directions, leading to equilibrium.
Equilibrium is reached when the rate of forward reaction equals the rate of reverse reaction.
Equilibrium does not mean equal concentrations of reactants and products, but rather stable concentrations where no further change occurs.
The equilibrium constant is defined as the ratio of the concentrations of products to reactants, raised to the power of their coefficients.
The equilibrium constant is only valid at a specific temperature.
The equilibrium constant allows prediction of resulting concentrations when reactant concentrations are changed.
Calculating equilibrium constant involves taking product concentrations raised to their respective coefficients and dividing by reactant concentrations raised to their respective coefficients.
The equilibrium constant provides insight into the direction of the reaction: <1 favors the reverse reaction, >1 favors the forward reaction.
Changing reactant concentrations alters the equilibrium constant, affecting the direction of the reaction.
Equilibrium constant calculations illustrate the relative abundance of reactants and products at equilibrium.
Adding reactants shifts the equilibrium towards product formation, and vice versa.
Understanding equilibrium constants aids in predicting reaction outcomes and optimizing reaction conditions.
The equilibrium constant remains constant regardless of the initial concentrations of reactants and products.
The concept of equilibrium allows for the study and manipulation of chemical reactions in a controlled manner.
Equilibrium constants are fundamental in chemical kinetics and thermodynamics, providing insights into reaction mechanisms and energetics.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: