18. Introduction to Chemical Equilibrium
TLDRThe video script is a transcript from an MIT OpenCourseWare lecture by Professor Catherine Drennan, focusing on the principles of chemical equilibrium. The lecture begins with a discussion on the importance of understanding chemistry to tackle global challenges, such as the Ebola crisis. It then delves into the concept of chemical equilibrium, emphasizing its dynamic nature where the rates of forward and reverse reactions are equal, resulting in no net change. Drennan explains the significance of the equilibrium constant (K) and how it relates to the Gibbs free energy (delta G), illustrating how a high K value indicates a greater amount of products at equilibrium. The lecture also covers the reaction quotient (Q) and how it predicts the direction a reaction will proceed to reach equilibrium. Drennan uses the example of the synthesis of ammonia from nitrogen and hydrogen to demonstrate these principles. Additionally, she touches on the application of chemical equilibrium in biological systems, such as the function of enzymes, and concludes with a real-world application of Le Chatelier's principle, highlighting its relevance beyond the classroom, even in business and life decisions.
Takeaways
- 🎓 The importance of understanding chemistry to solve global challenges, such as the energy problem and health crises like Ebola, is emphasized.
- 📚 MIT OpenCourseWare provides free educational resources, and donations support the continuation of this service.
- 📈 The concept of Gibbs free energy (delta G) is introduced, explaining how it can be negative, indicating a spontaneous process, or positive, indicating a non-spontaneous process.
- 🔁 Chemical equilibrium is described as a dynamic process where the rates of the forward and reverse reactions are equal, resulting in no net change.
- 🧪 The equilibrium constant (K) is explained as the ratio of products to reactants at equilibrium, and how it can be used to predict the direction of a reaction.
- 🌡️ The effect of temperature on chemical equilibrium is discussed, noting that an increase in temperature can shift the equilibrium position.
- 📊 The relationship between delta G, delta G0, and the reaction quotient (Q) is detailed through the equation delta G = delta G0 + RT ln(Q/K).
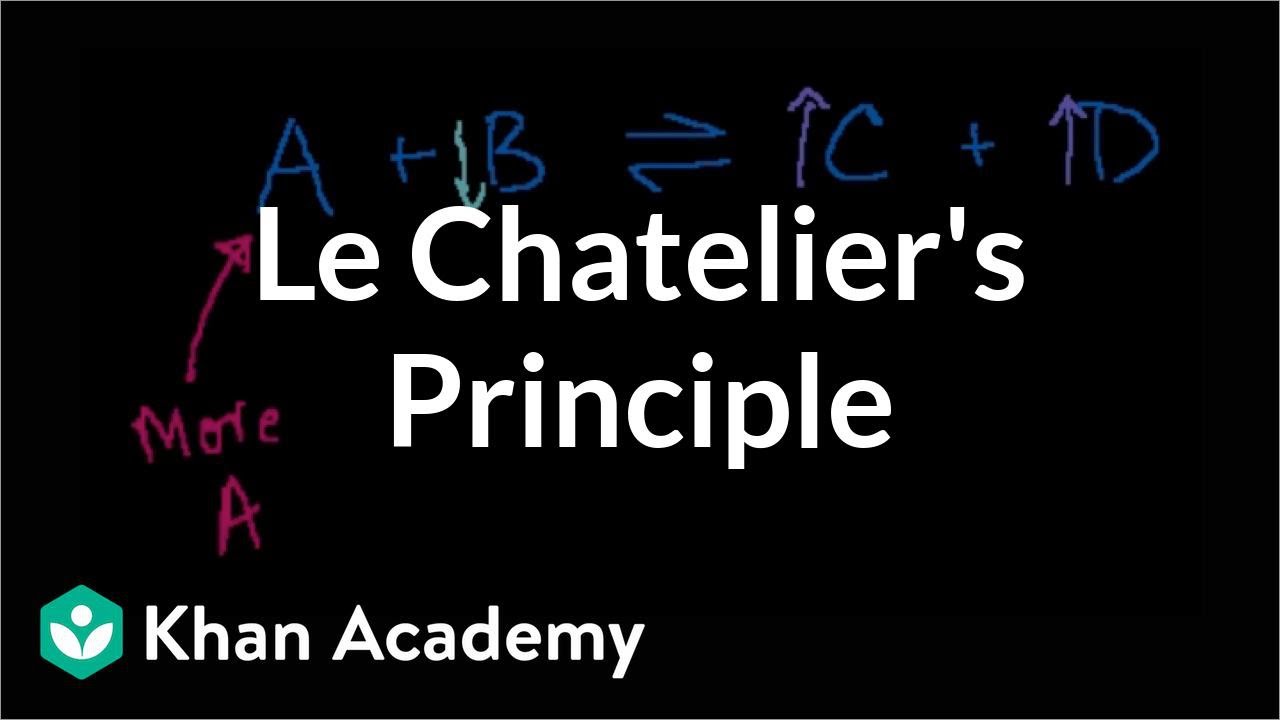
- ⚖️ Le Chatelier's principle is introduced, stating that a system at equilibrium will adjust to minimize the effect of any stress applied to it.
- 🧵 The example of the transformation between active and inactive states of the enzyme Ribonucleotide Reductase (RNR) is used to illustrate the concept of chemical equilibrium in biological systems.
- 🤔 The significance of being able to balance chemical equations is highlighted, as it is necessary for understanding and calculating chemical equilibria.
- 📏 The concept of significant figures in calculations is discussed, with an emphasis on their importance in scientific notation and calculations.
- 🎯 The goal of education is highlighted as aiming for an average grade close to 90% to demonstrate excellent knowledge of chemistry among students.
Q & A
What is the significance of the MIT chemistry bag mentioned in the transcript?
-The MIT chemistry bag is a special item that signifies achievement or participation in an MIT chemistry course or event, indicating a connection to the educational institution and its chemistry program.
How does the concept of delta H and delta S help in determining the spontaneity of a reaction?
-The values of delta H (change in enthalpy) and delta S (change in entropy) are crucial in determining whether a reaction is spontaneous. A negative delta H and a positive delta S generally indicate a spontaneous process. The sign of delta G, which is dependent on delta H, delta S, and temperature (T), ultimately determines spontaneity.
What does Professor Drennan aim for in terms of class performance on exams?
-Professor Drennan aims for the class average to be close to 90, demonstrating excellent knowledge of chemistry. She designs exams to reflect that a student with excellent chemistry knowledge should be able to achieve a score of 90% or above.
How does the concept of chemical equilibrium relate to the real-world challenges we face, such as the Ebola situation?
-Chemical equilibrium is fundamental to understanding how reactions proceed and how they can be manipulated. This knowledge is essential for tackling real-world challenges, including health crises like Ebola, where chemical processes are critical for developing treatments and vaccines.
What is the relationship between the reaction quotient (Q) and the equilibrium constant (K)?
-The reaction quotient (Q) is the ratio of products to reactants at any given time during a reaction, while the equilibrium constant (K) is the ratio of products to reactants at equilibrium. If Q is less than K, the reaction will proceed spontaneously in the forward direction to produce more products. Conversely, if Q is greater than K, the reaction will proceed in the reverse direction to use up excess products.
What is the significance of the principle of Le Chatelier in predicting the direction of a reaction under stress?
-Le Chatelier's principle states that if a system at equilibrium is subjected to a change in conditions, the system will adjust to counteract the change and re-establish equilibrium. This principle is used to predict how a reaction will shift in response to changes such as added reactants, removed products, changes in temperature, or pressure.
How does the stoichiometry of a reaction affect the calculation of the equilibrium constant (K)?
-The stoichiometry of a reaction determines the coefficients of the balanced chemical equation, which in turn affects the calculation of K. The concentrations or partial pressures of reactants and products in the expression for K are raised to the power of their stoichiometric coefficients, reflecting the proportions in which they participate in the reaction.
What is the role of the Gibbs free energy (delta G) in determining the spontaneity of a reaction at a given point?
-The Gibbs free energy (delta G) is a thermodynamic potential that measures the maximum reversible work that a system can perform at constant temperature and pressure. A negative delta G indicates a spontaneous process, while a positive delta G means the reaction is non-spontaneous under the current conditions.
Why is it important to understand the difference between the reaction quotient (Q) and the equilibrium constant (K)?
-Understanding the difference between Q and K is essential for determining the direction in which a reaction will proceed to reach equilibrium. Q is used to calculate the current state of the reaction, while K represents the state of the reaction at equilibrium. By comparing Q to K, one can predict whether the reaction will produce more products (Q < K) or more reactants (Q > K) to achieve equilibrium.
How does the value of K relate to the stability of the products in a reaction at equilibrium?
-A large value of K indicates that the reaction strongly favors the formation of products at equilibrium, suggesting that the products are more stable under the given conditions. Conversely, a small value of K implies that the reaction favors the reactants, indicating that the products are less stable relative to the reactants.
What is the relevance of chemical equilibrium in biological systems, such as the study of enzymes?
-Chemical equilibrium is highly relevant in biological systems as it governs the behavior of enzymes and other biological catalysts. Enzymes often exist in an equilibrium between active and inactive states, which is crucial for regulating metabolic pathways. Understanding this equilibrium can help in the development of drugs targeting specific enzymes, such as in cancer treatment or antibacterial therapies.
Outlines
📚 Introduction to MIT OpenCourseWare and Chemistry Class
The video begins with an introduction to MIT OpenCourseWare, which provides free educational resources under a Creative Commons license. The audience is encouraged to donate to support this initiative. Professor Catherine Drennan then engages with the audience about a chemistry problem, discussing the concept of delta H and delta S, and how temperature affects these values. She emphasizes the importance of understanding chemistry to tackle global challenges, such as the energy crisis and health issues, including the Ebola situation.
🎓 Exam Performance and Upcoming Curriculum
Professor Drennan discusses the performance of her students on exam 2, with an average score of 84.7, and expresses her desire for the class average to be closer to 90. She mentions the next topics to be covered, including thermodynamics, chemical equilibrium, and acid-base chemistry. The lecture also covers the concept of entropy with a humorous anecdote about her T-shirts being related to the topic.
🔁 Understanding Chemical Equilibrium
The lecture delves into the concept of chemical equilibrium, explaining that it is a dynamic process where the rates of the forward and reverse reactions are equal, resulting in no net change. Drennan uses the analogy of work-life balance to help students understand the concept. She also discusses how to represent equilibrium with reaction quotients (Q) and equilibrium constants (K), and how these values are used to determine the direction of a reaction.
🔢 Calculating Reaction Quotients and Equilibrium Constants
The video explains how to calculate the reaction quotient Q and the equilibrium constant K using the concentrations or partial pressures of reactants and products. It covers the relationship between Q, K, and the Gibbs free energy (delta G), and how these values can predict the direction of a reaction. The importance of balancing chemical equations for these calculations is emphasized.
🧪 Application of Le Chatelier's Principle
Le Chatelier's principle is introduced, stating that a system in equilibrium will adjust to minimize the effect of any changes made to the system. This principle is illustrated with examples, such as adding or removing reactants or products, and how these actions affect the position of equilibrium. The concept is also related to real-life situations, encouraging students to apply the principle of minimizing stress in their lives.
🍞 Example: Baking Soda and Chemical Equilibrium
An example involving baking soda is used to demonstrate the application of chemical equilibrium principles. The reaction of baking soda to produce CO2 gas is discussed, and how the equilibrium constant K and delta G0 values can predict the amount of product formed at different temperatures. This ties into the practical application of chemical equilibrium in everyday processes, such as baking.
🤔 Stress and Equilibrium in Biological Systems
The lecture concludes with a discussion on how chemical equilibrium principles apply to biological systems, such as enzymes. A video featuring Nozomi Ando, an MIT graduate and chemistry professor at Princeton University, explains her research on a protein called Ribonucleotide Reductase, which plays a crucial role in DNA synthesis and repair. The equilibrium between active and inactive states of the enzyme is regulated by cellular components, highlighting the importance of understanding chemical equilibrium in biological processes.
📈 Applying Le Chatelier's Principle to Stressful Conditions
The final part of the lecture focuses on applying Le Chatelier's principle to predict the direction of reactions under stress, such as changes in temperature, pressure, or concentration of reactants and products. The principle is illustrated with the example of the synthesis of ammonia from nitrogen and hydrogen, showing how adding or removing reactants or products affects the equilibrium position. The lecture ends with a reminder for students to complete their problem set and a humorous analogy relating to student life and stress management.
Mindmap
Keywords
💡Chemical Equilibrium
💡Gibbs Free Energy (ΔG)
💡Reaction Quotient (Q)
💡Equilibrium Constant (K)
💡Le Chatelier's Principle
💡Standard Gibbs Free Energy (ΔG°)
💡Stoichiometry
💡Thermodynamics
💡Enthalpy (ΔH)
💡Entropy (ΔS)
💡Exothermic and Endothermic Reactions
Highlights
MIT OpenCourseWare offers high-quality educational resources for free, supported by donations.
An MIT chemistry bag is presented as a special item related to the course.
The concept of delta H as a y-intercept and its relation to the slope of the reaction is discussed.
The importance of temperature in affecting the change from a negative to a positive delta G is highlighted.
The average score for exam 2 was 84.7, reflecting the class's performance.
The goal is set for the average score of exam 3 to be back at 87, aiming for an overall average close to 90.
Chemistry knowledge is emphasized as crucial for tackling future challenges, such as the Ebola situation.
Thermodynamics, chemical equilibrium, and acid-base are the main topics for the next exam.
Problem set 5 focuses on thermodynamics with a shorter duration due to fewer days allocated for completion.
The concept of entropy is humorously related to the shipping mishaps of a T-shirt order.
Chemical equilibrium is described as a calming but dynamic process where the rates of forward and reverse reactions are equal.
The example of nitrogen and hydrogen forming ammonia is used to illustrate the concept of chemical equilibrium.
The relationship between delta G, delta G0, and the reaction quotient Q is detailed with an equation.
The equilibrium constant K is defined as the ratio of products to reactants at equilibrium.
The principle of Le Chatelier is introduced, stating that a system in equilibrium will react to minimize stress.
The impact of temperature on the equilibrium constant K for the reaction of N2 and H2 to form ammonia is discussed.
The significance of the equilibrium constant K in predicting the direction of a reaction and its industrial applications is explained.
Le Chatelier's principle is applied to real-life scenarios, including personal stress management and professional problem-solving.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: