Chemical Equilibrium Full Topic Video
TLDRThe video script delves into the concept of chemical equilibrium, explaining the dynamics of reactions and the factors affecting them, such as concentration, pressure, volume, and temperature. It introduces the equilibrium constant (K) and its relationship with the Gibbs free energy, highlighting the importance of understanding how changes in conditions can shift the equilibrium. The script also discusses the application of Le Chatelier's principle in predicting the direction of equilibrium shifts and its implications for maximizing product formation in chemical reactions. Additionally, it provides practical examples and calculations to illustrate these concepts, emphasizing the significance of equilibrium in chemical processes.
Takeaways
- 📚 Chemical equilibrium is a state where the rates of the forward and backward reactions are equal, resulting in constant concentrations of reactants and products.
- 🔄 Reversible reactions involve reactants converting to products and vice versa, with the system eventually reaching a dynamic balance.
- 📊 The equilibrium constant (K) is expressed as the product of the concentrations (for KC) or pressures (for KP) of the products raised to their stoichiometric coefficients, divided by the same for the reactants.
- 🌡️ Changes in temperature, concentration, pressure, and volume can affect the position of equilibrium, as described by Le Chatelier's Principle.
- 📈 The direction of a reaction at equilibrium can be predicted based on the relative values of the reaction quotient (Q) and the equilibrium constant (K).
- 🔧 The magnitude of K indicates the extent of product formation; a large K value suggests a greater concentration of products compared to reactants.
- 🌟 Homogeneous equilibrium occurs when all reactants and products are in the same phase, such as all being gases or all being in solution.
- 🛠️ The ICE (Initial, Change, Equilibrium) table is a tool used to determine the initial concentrations, changes in concentration, and equilibrium concentrations of reactants and products.
- 📝 When calculating K, only equilibrium concentrations are used; initial concentrations or concentrations at any other point in the reaction are not included.
- 🔄 The relationship between KP and KC can be derived from the ideal gas law and is given by KP = KC * (RT)^Δn, where Δn is the change in the number of moles of gas.
- 🔧 The concept of chemical equilibrium is crucial in understanding how reactions behave under different conditions and how to manipulate these conditions to favor desired outcomes.
Q & A
What is the main concept of chemical equilibrium?
-Chemical equilibrium refers to the state where the rate of the forward reaction equals the rate of the backward reaction, resulting in constant concentrations of reactants and products.
What are the two types of equilibrium mentioned in the script?
-The two types of equilibrium mentioned are homogeneous equilibrium, where all reactants and products are in the same phase, and heterogeneous equilibrium, where the reactants and products are in different phases.
How is the equilibrium constant (K) expressed for gaseous reactions?
-For gaseous reactions, the equilibrium constant K can be expressed as KC, which is based on concentrations, or KP, which is based on partial pressures. KC is calculated as the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients. KP is calculated similarly, but with pressures of the gaseous reactants and products instead of concentrations.
What is the ICE table used for in the context of chemical equilibrium?
-The ICE table, which stands for Initial, Change, and Equilibrium, is used to determine the initial concentrations, the changes in concentration as the reaction proceeds, and the equilibrium concentrations of reactants and products.
How does the presence of solids and liquids affect the expression of the equilibrium constant K?
-Solids and liquids do not appear in the expressions for the equilibrium constant K because they are considered pure substances and their concentrations are taken to be one. Adding or multiplying by one does not change the value, so they are omitted from the expression.
What is the relationship between the equilibrium constant K and the Gibbs free energy?
-At equilibrium, the nonstandard Gibbs free energy is zero, and the relationship between the standard Gibbs free energy and K is given by the equation: standard Gibbs free energy = -RT ln(K), where R is the gas constant, T is the temperature in Kelvin, and ln(K) is the natural logarithm of the equilibrium constant K.
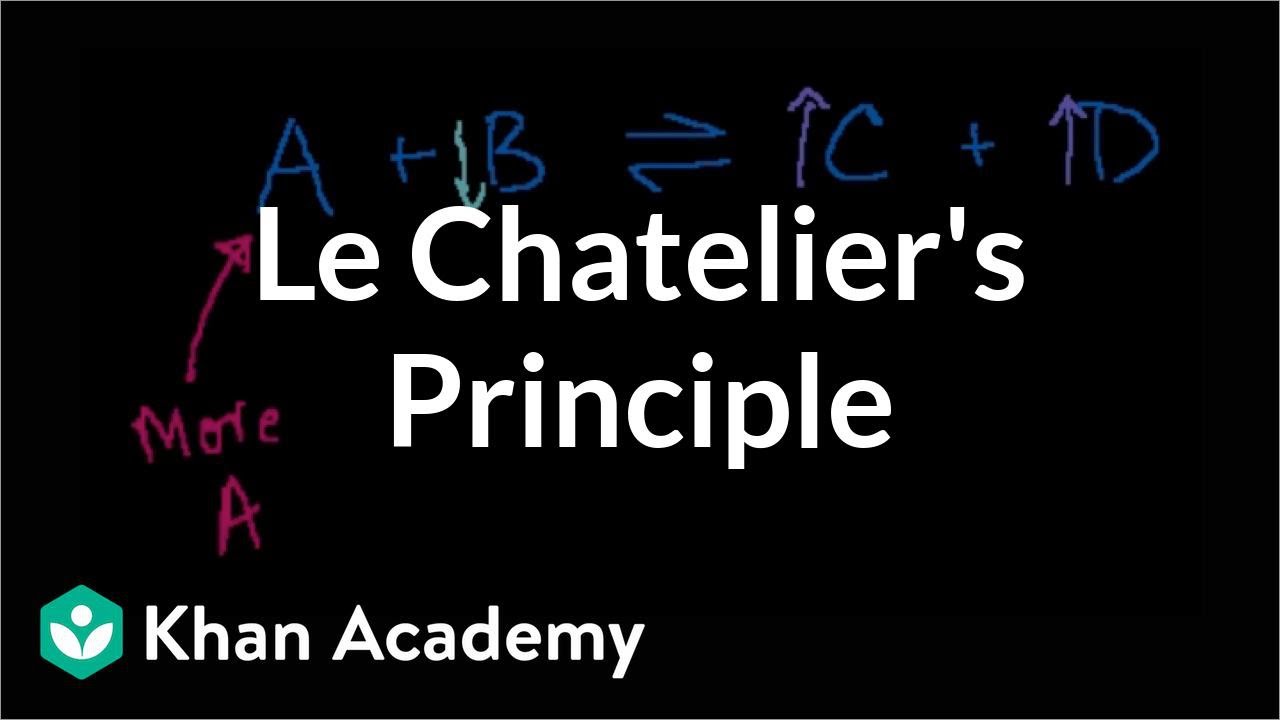
What is Le Chatelier's Principle and how does it apply to chemical equilibrium?
-Le Chatelier's Principle states that if a system at equilibrium is subjected to a change in concentration, pressure, temperature, or volume, the system will adjust itself to counteract the change and re-establish equilibrium. For example, if the concentration of a reactant is increased, the system will shift to favor the forward reaction to consume the added reactant and restore balance.
How does the volume of a container affect the position of equilibrium for gaseous reactions?
-For gaseous reactions, changing the volume of the container affects the pressure. Increasing the volume (and thus decreasing the pressure) will favor the side of the reaction with more moles of gas, while decreasing the volume (and thus increasing the pressure) will favor the side with fewer moles of gas.
What is the effect of temperature on the direction of a chemical reaction at equilibrium?
-The effect of temperature on the direction of a chemical reaction at equilibrium depends on whether the reaction is endothermic (absorbs heat) or exothermic (releases heat). Increasing the temperature will favor the endothermic reaction (shift the equilibrium towards the products), while decreasing the temperature will favor the exothermic reaction (shift the equilibrium towards the reactants).
How does the addition of a catalyst affect the equilibrium of a reaction?
-Adding a catalyst to a reaction increases the rate at which equilibrium is achieved, but it does not change the position of the equilibrium or the equilibrium constant. A catalyst equally affects the forward and reverse reaction rates, so the concentrations of reactants and products at equilibrium remain unchanged.
What is the relationship between the equilibrium constant K and the reaction quotient Q?
-The equilibrium constant K is the value of the reaction quotient Q at the point of equilibrium. When Q is greater than K, the reaction will shift towards the reactants to decrease Q, and when Q is less than K, the reaction will shift towards the products to increase Q.
Outlines
📘 Introduction to Chemical Equilibrium
This paragraph introduces the concept of chemical equilibrium, emphasizing the importance of understanding reversible reactions. It explains that at equilibrium, the rate of the forward and backward reactions are equal, resulting in constant concentrations of reactants and products. The paragraph also introduces the representation of reactants and products in chemical equations and discusses how the concentration of these substances changes over time as they reach equilibrium.
📊 Calculations of Equilibrium Constants
This section delves into the calculations of equilibrium constants (K), distinguishing between homogeneous and heterogeneous equilibria. It explains how to express the equilibrium constant (K) using concentrations (Kc) and pressures (Kp), and how these expressions account for the coefficients in the balanced chemical equation. The paragraph also highlights the exclusion of solids and liquids from K expressions, as their concentrations are considered constant.
🧪 Practical Example of Equilibrium Constant Calculations
The paragraph presents a practical example to illustrate the calculation of equilibrium constants. It walks through the process of writing the K expression for a given balanced chemical equation, emphasizing the use of stoichiometric coefficients and the distinction between reactants and products. The example also clarifies how to handle different phases of reactants and products in the K expression.
📉 Ice Table Method for Equilibrium Calculations
This part introduces the ice table method, a tool for determining initial and equilibrium concentrations in a chemical reaction. The ice table helps to visualize the changes in concentration as the reaction progresses towards equilibrium. The paragraph explains how to use the ice table to calculate the equilibrium constant (Kc) and provides a step-by-step approach to solving equilibrium problems using this method.
🔄 Relationship Between K, KP, and Gibbs Free Energy
This section explores the relationship between the equilibrium constants K and KP, and how they relate to Gibbs free energy. It explains the concept of standard and non-standard Gibbs free energy, and how the value of K can be calculated from the standard Gibbs free energy and temperature. The paragraph also discusses the direction of reaction at equilibrium and how changes in concentration, pressure, and temperature can shift the equilibrium position, according to Le Chatelier's principle.
🌡️ Effects of Changes in Reaction Conditions on Equilibrium
The paragraph discusses how various changes in reaction conditions, such as concentration, pressure, volume, and temperature, can affect the position of equilibrium. It explains Le Chatelier's principle in detail, stating that a system at equilibrium will adjust to counteract any changes or disturbances applied to it. The effects of increasing or decreasing these conditions are discussed in relation to both endothermic and exothermic reactions, providing a comprehensive understanding of how equilibrium shifts in response to external changes.
Mindmap
Keywords
💡Chemical Equilibrium
💡Reversible Reactions
💡Equilibrium Constant (K)
💡Stoichiometric Coefficients
💡Concentration
💡Le Chatelier's Principle
💡Gibbs Free Energy
💡Ideal Gas Law
💡Partial Pressures
💡Dynamic Equilibrium
💡Equilibrium Table (ICE Table)
Highlights
Introduction to chemical equilibrium and its importance in understanding reversible reactions.
Explanation of how to determine when a reaction has reached chemical equilibrium based on the rates of the forward and backward reactions.
Discussion on the concept of homogeneous equilibrium and its occurrence when all reactants and products are in the same phase.
Elucidation on the calculation of the equilibrium constant (K) and its dependence on the concentrations or partial pressures of reactants and products.
Explanation of how to express the equilibrium constant (K) for gaseous reactants and products using stoichiometric coefficients.
Clarification that solids and liquids do not appear in the equilibrium constant expressions, as their concentrations are considered to be one.
Practical example demonstrating how to write the equilibrium constant expression for a given chemical equation.
Introduction to the ICE (Initial, Change, Equilibrium) table and its use in determining initial concentrations, changes in concentration, and equilibrium concentrations.
Explanation of how to use the ICE table to calculate the equilibrium constant (K) at a given temperature based on equilibrium concentrations.
Discussion on the relationship between the equilibrium constant (K) and the Gibbs free energy, highlighting how K can be calculated from standard Gibbs free energy values.
Illustration of how to find the equilibrium concentration of a product using the ICE table and given equilibrium constant (K).
Explanation of the relationship between the equilibrium constant (KP) and (KC), and how changes in pressure and temperature affect the equilibrium constant.
Practice questions to reinforce understanding of chemical equilibrium, calculation of K, and application of Le Chatelier's principle.
Application of Le Chatelier's principle to predict the direction of shift in equilibrium when various stresses such as changes in concentration, pressure, and temperature are applied.
Discussion on the effects of adding or removing reactants or products on the equilibrium and how the system adjusts to re-establish equilibrium.
Explanation of how the number of moles of gases affects the equilibrium position, particularly in relation to volume and pressure changes.
Overview of how temperature changes can influence the direction of a reaction at equilibrium, with specific reference to endothermic and exothermic reactions.
Concluding remarks on the practical applications of understanding chemical equilibrium, K calculations, and the impact of various stresses on maintaining equilibrium.
Transcripts
Browse More Related Video

ATI TEAS 7 I Complete Chemistry Review Part 2 I

AP Chemistry Unit 7 Practice Problems 2020

18. Introduction to Chemical Equilibrium

Reactions in equilibrium | Chemical equilibrium | Chemistry | Khan Academy

Le Chatelier's Principle
Le Chatelier's principle | Chemical equilibrium | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: