Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
TLDRThe video script discusses the concept of chemical equilibrium, explaining it as a dynamic state where the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products. It uses an analogy of car traffic between two cities to illustrate this balance. The script further explains how to represent equilibrium graphically using concentration profiles and reaction rates over time. It also covers the calculation of the equilibrium constant (K), distinguishing between Kc for concentration and Kp for partial pressure, and provides examples of how to calculate these constants given different reaction scenarios.
Takeaways
- 🌟 Chemical equilibrium refers to the state where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in constant concentrations of reactants and products.
- 🚗 The analogy of car traffic between two cities demonstrates how equilibrium works, with the flow of cars representing the dynamic nature of chemical reactions at equilibrium.
- 📊 A concentration profile graph illustrates the changes in concentration of reactants and products over time, showing that at equilibrium, the concentrations stabilize and no longer change.
- 📈 The rate of the forward reaction decreases as the concentration of reactants decreases, while the rate of the reverse reaction increases as the concentration of products increases.
- 🔄 At equilibrium, the concentrations of reactants and products remain constant because the rates of the forward and reverse reactions are equal, demonstrating a dynamic equilibrium.
- 📝 The equilibrium constant (Kc) is the ratio of the concentration of products to the concentration of reactants, raised to the power of their respective stoichiometric coefficients.
- 📐 The equilibrium expression can be written using the law of mass action, with the concentrations of reactants and products at equilibrium substituted into the expression to find Kc or Kp.
- 🔢 To calculate the equilibrium constant (Kc or Kp), the balanced chemical equation and the given equilibrium concentrations or pressures are used, along with the stoichiometric coefficients.
- ⚖️ The relationship between Kc and Kp is given by the formula Kp = Kc * (RT/V)^Δn, where Δn is the difference in the sum of the stoichiometric coefficients of the products and reactants.
- 🔄 Adjusting the coefficients in a balanced chemical equation affects the equilibrium constant (K) by squaring, cubing, or taking the square root of the original K value, depending on the change.
- 🧪 Practice problems in the script demonstrate how to calculate Kc and Kp, as well as how to find the equilibrium concentrations or pressures for reactants and products given an initial condition and the equilibrium constant.
Q & A
What is the definition of chemical equilibrium?
-Chemical equilibrium occurs when the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in constant concentrations of the reactants and products over time, indicating no net change in the system.
How is equilibrium different from a static state?
-Equilibrium is a dynamic state where reactions are still occurring, with reactants converting to products and vice versa, but the concentrations of the reactants and products remain constant because the rates of the forward and reverse reactions are equal.
What does it mean for the concentrations of reactants and products to be constant at equilibrium?
-At equilibrium, the concentrations of reactants and products are constant because the rate of the forward reaction (converting reactants to products) is equal to the rate of the reverse reaction (converting products back to reactants), resulting in no net change in the system.
How can the concept of equilibrium be visually represented?
-The concept of equilibrium can be visually represented using a concentration profile graph, where the concentration of reactants is plotted on the y-axis and time on the x-axis. At equilibrium, the concentration of reactants and products becomes horizontal, indicating no change over time.
What is the relationship between the forward and reverse reaction rates at equilibrium?
-At equilibrium, the rate of the forward reaction (the conversion of reactants to products) is equal to the rate of the reverse reaction (the conversion of products back to reactants).
What is the equilibrium constant (k) and how is it calculated?
-The equilibrium constant (k) is a measure of the extent of a reaction at equilibrium. It is calculated as the ratio of the concentration of products to the concentration of reactants, with each concentration raised to the power of its stoichiometric coefficient in the balanced chemical equation.
What are the two types of equilibrium constants and how do they differ?
-The two types of equilibrium constants are kc and kp. kc is the equilibrium concentration constant and is associated with the concentrations of reactants and products in molarity. kp is the equilibrium partial pressure constant and is associated with the partial pressures of gases involved in the reaction.
How can you calculate the equilibrium constant (k) for a given reaction?
-To calculate the equilibrium constant (k) for a given reaction, you need to know the equilibrium concentrations or partial pressures of the reactants and products. Then, you can use the law of mass action to express k as the product of the concentrations or partial pressures of the products raised to their stoichiometric coefficients, divided by the same for the reactants.
What is the ICE table and how is it used in calculating equilibrium concentrations?
-The ICE table (Initial, Change, Equilibrium) is a method used to calculate the equilibrium concentrations of reactants and products in a chemical reaction. It helps to keep track of the changes in concentration as the reaction proceeds towards equilibrium. The table lists the initial concentrations, the changes that occur as the reaction proceeds, and the final equilibrium concentrations.
How does the value of the equilibrium constant (k) change when the reaction is adjusted by altering the coefficients?
-When the reaction is adjusted by altering the coefficients, the value of the equilibrium constant (k) changes according to the law of mass action. If the coefficients are doubled, the new equilibrium constant (k') will be the square of the original k. If the coefficients are halved, k' will be the square root of the original k. If the reaction is reversed, k' will be the reciprocal of the original k.
How can you relate the equilibrium constants kp and kc for the same reaction?
-The relationship between the equilibrium constants kp and kc for the same reaction can be established using the ideal gas law (PV=nRT). By replacing the partial pressures in the expression for kp with concentrations (using P=cRT), you can derive an equation that relates kp to kc. Specifically, kp = kc * (RT/Δn)^Δn, where Δn is the difference between the sum of the stoichiometric coefficients of the products and those of the reactants.
Outlines
🌟 Introduction to Chemical Equilibrium
This paragraph introduces the concept of equilibrium in the context of chemistry, focusing on reversible reactions where a substance 'a' can convert to 'b' and vice versa. Equilibrium is achieved when the forward and reverse reaction rates are equal, resulting in constant concentrations of 'a' and 'b'. This state is referred to as dynamic equilibrium, as the reactions continue to occur despite the system being at equilibrium. The analogy of cars moving between two cities is used to illustrate this concept, emphasizing that equilibrium does not imply a static state but rather a balanced dynamic process.
📈 Visual Representation of Equilibrium
The paragraph explains how to visually represent the concept of equilibrium using a concentration profile graph. The graph has the concentration of reactants on the y-axis and time on the x-axis. It describes a scenario where 'a' is initially present and 'b' is absent, and as the reaction proceeds, the concentration of 'a' decreases while that of 'b' increases until they reach a constant value, indicating equilibrium. The point where the concentration graph becomes horizontal signifies that the forward and reverse reaction rates are equal. The paragraph also introduces the concept of plotting the rates of forward and reverse reactions against time, showing how these rates change and eventually equalize at equilibrium.
🧪 Practice Problems and Equilibrium Constants
This section delves into practice problems related to equilibrium constants, distinguishing between 'kc' and 'kp'. 'Kc' is the equilibrium concentration constant, while 'kp' is associated with partial pressure. The law of mass action is introduced as a basis for writing equilibrium expressions, with coefficients becoming exponents in these expressions. The paragraph presents a series of problems that involve writing equilibrium expressions for given reactions and calculating equilibrium constants using provided concentrations or pressures at equilibrium.
🔢 Calculation of Equilibrium Constants
The paragraph focuses on calculating the equilibrium constant 'kc' using given concentrations of reactants and products at equilibrium. It provides a step-by-step approach to finding the balanced chemical equation, applying the law of mass action, and using equilibrium concentrations to determine the value of 'kc'. The example given involves nitrogen reacting with chlorine to form nitrogen trichloride, with specific concentrations provided for the gases at equilibrium. The process of calculating 'kc' is clearly outlined, emphasizing the importance of using equilibrium concentrations in these calculations.
🌡️ Relationship Between 'Kc' and 'Kp'
This part of the script discusses the relationship between 'kc' and 'kp', the equilibrium constants based on concentration and partial pressure, respectively. It introduces a formula that relates 'kc' and 'kp' through the ideal gas constant 'R' and temperature 'T', explaining how to convert between these two constants. The concept of 'delta n', the difference between the sum of coefficients of the products and reactants, is also introduced. The paragraph provides a method for calculating 'kp' from 'kc' and vice versa, using the provided formula and the value of 'delta n' for the reaction.
🧠 Understanding Changes in Reaction Coefficients
The paragraph explores how changes in the coefficients of a chemical reaction affect the equilibrium constant 'k'. It explains that if the coefficients are doubled, the new equilibrium constant 'k prime' will be the square of the original 'k'. Conversely, if the coefficients are halved, 'k prime' will be the square root of the original 'k'. The paragraph also discusses what happens if the reaction is reversed, in which case 'k prime' will be the reciprocal of 'k'. These principles are demonstrated with examples, showing how to calculate the new 'k' value for adjusted reactions.
📝 ICE Table and Equilibrium Calculations
The paragraph describes the use of the ICE (Initial, Change, Equilibrium) table for calculating equilibrium concentrations in a reaction. It provides a step-by-step guide on how to set up the ICE table, how the reaction direction is determined based on initial conditions, and how to calculate changes in concentrations as the reaction proceeds towards equilibrium. The example given involves the decomposition of ammonia into nitrogen and hydrogen gases, with initial and equilibrium pressures provided. The paragraph demonstrates how to use the ICE table to find the equilibrium partial pressures of the reactants and products.
📊 Solving for Equilibrium Partial Pressures
This section focuses on solving for equilibrium partial pressures in a reaction. It continues the example from the previous paragraph, using the ICE table to determine the direction of the reaction and the changes in concentrations of reactants and products. The paragraph explains how to use the equilibrium partial pressures of products and the initial partial pressure of the reactant to calculate the equilibrium partial pressure of another reactant. It also shows how to calculate the equilibrium constant 'kp' for the reaction, using the equilibrium partial pressures at equilibrium.
Mindmap
Keywords
💡Equilibrium
💡Concentration Profile
💡Dynamic Equilibrium
💡Rate Constants
💡Equilibrium Constant (K)
💡Law of Mass Action
💡Stoichiometric Coefficients
💡Reversible Reaction
💡Concentration
💡Partial Pressure
💡ICE Table
Highlights
Equilibrium is defined as the state where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in constant concentrations of reactants and products.
Chemical equilibrium is a dynamic state, meaning reactions continue to occur, but with net changes in concentrations.
An analogy of two cities with a highway connecting them illustrates the concept of equilibrium through the exchange of cars between the cities.
The concentration profile graph demonstrates how the concentrations of reactants and products change over time until they reach equilibrium.
At equilibrium, the rate of the forward reaction equals the rate of the reverse reaction, and the concentrations of reactants and products stabilize.
The equilibrium constant (K) is the ratio of the forward rate constant to the reverse rate constant and is represented by the concentrations of products over reactants.
The law of mass action is used to write the equilibrium expressions for both concentration (Kc) and partial pressure (Kp) constants.
The coefficients in a balanced chemical equation become exponents in the equilibrium expression, reflecting the relationship between reactants and products at equilibrium.
Practice problems are provided to demonstrate how to write equilibrium expressions and calculate the equilibrium constant for different types of reactions.
The ideal gas law (PV=nRT) is used to relate partial pressures to concentrations, allowing for the calculation of both Kc and Kp from given data.
The relationship between Kc and Kp can be expressed as Kp = Kc * (RT/P)^Δn, where Δn is the difference in the sum of the stoichiometric coefficients of the products and reactants.
Adjusting the coefficients in a balanced chemical equation affects the equilibrium constant (K) by raising it to the power corresponding to the change in coefficients.
Reversing a reaction changes the equilibrium constant (K) to its reciprocal, demonstrating the inverse relationship between the forward and reverse reactions at equilibrium.
The Initial Concentration Equation (ICE) table is a useful tool for determining changes in concentrations and equilibrium positions for reactions in a closed system.
By using the ICE table and the equilibrium constant, one can calculate the equilibrium concentrations or partial pressures of reactants and products in a chemical reaction.
The concept of equilibrium is not limited to chemistry; it can also be applied to various real-world scenarios, such as population dynamics or economic systems.
Understanding and applying chemical equilibrium principles are crucial for various fields, including industrial processes, environmental studies, and pharmaceutical development.
The video provides a comprehensive overview of chemical equilibrium, including its definition, dynamics, and applications, offering valuable insights for students and professionals alike.
Transcripts
Browse More Related Video
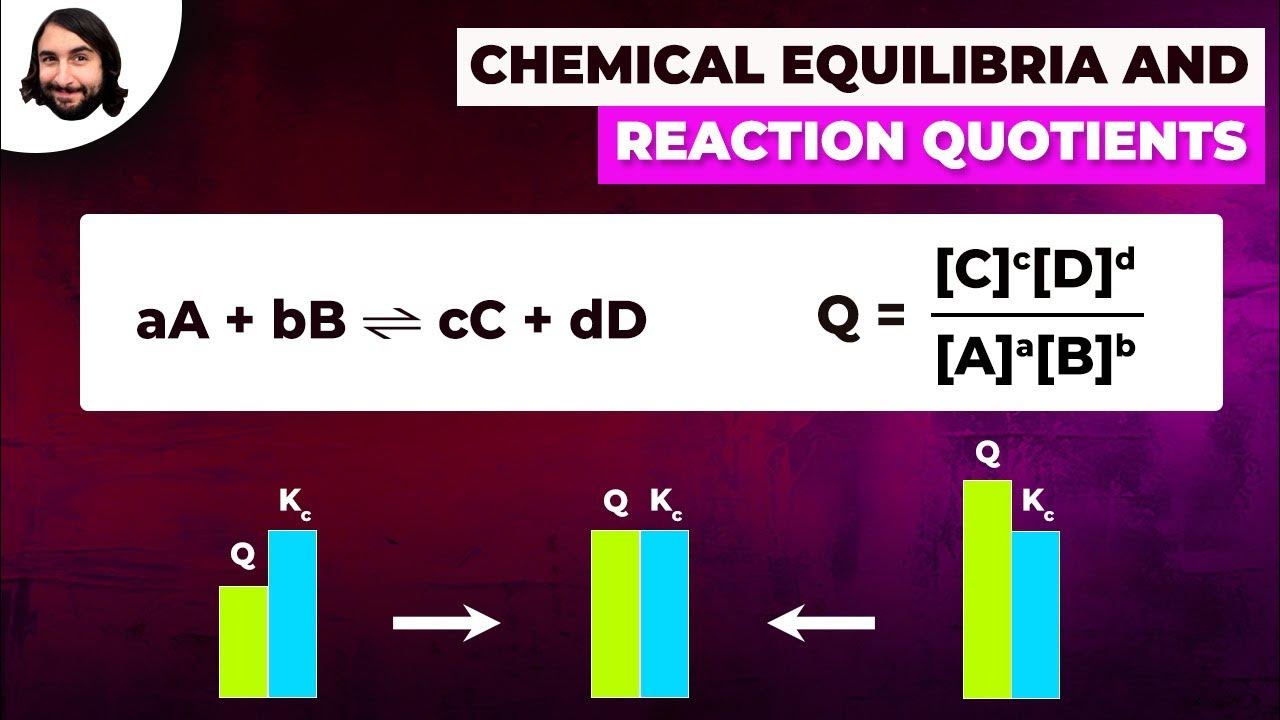
Chemical Equilibria and Reaction Quotients

How To Calculate Kp From Kc - Chemical Equilibrium

15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
What Is Equilibrium? AP Chemistry Unit 7, Topic 1 Daily Video
Le Chatelier's principle | Chemical equilibrium | Chemistry | Khan Academy

AP Chem - Unit 7 Review - Equilibrium in 10 Minutes - 2023
5.0 / 5 (0 votes)
Thanks for rating: