Chemical Equilibria and Reaction Quotients
TLDRIn this video, Professor Dave delves into the concept of chemical equilibria, explaining that reversible reactions can reach a state where the rates of forward and reverse reactions are equal, resulting in a dynamic equilibrium. He introduces the ICE (Initial, Change, Equilibrium) table method to calculate the concentrations of reactants and products at equilibrium, and discusses the significance of the equilibrium constant (Kc) in determining whether a reaction favors products or reactants. The video also covers how to use Kc to predict the direction of a reaction given non-equilibrium concentrations through the reaction quotient (Q). The tutorial is capped off with a more complex ICE table example, illustrating how to solve for equilibrium concentrations using stoichiometry and the equilibrium expression. The engaging presentation encourages viewers to subscribe for more educational content and reach out with questions.
Takeaways
- 🔁 **Reversible Reactions**: Chemical reactions can be reversible, meaning products can revert to reactants.
- 🌀 **Dynamic Equilibrium**: A system is at dynamic equilibrium when the rates of the forward and reverse reactions are equal.
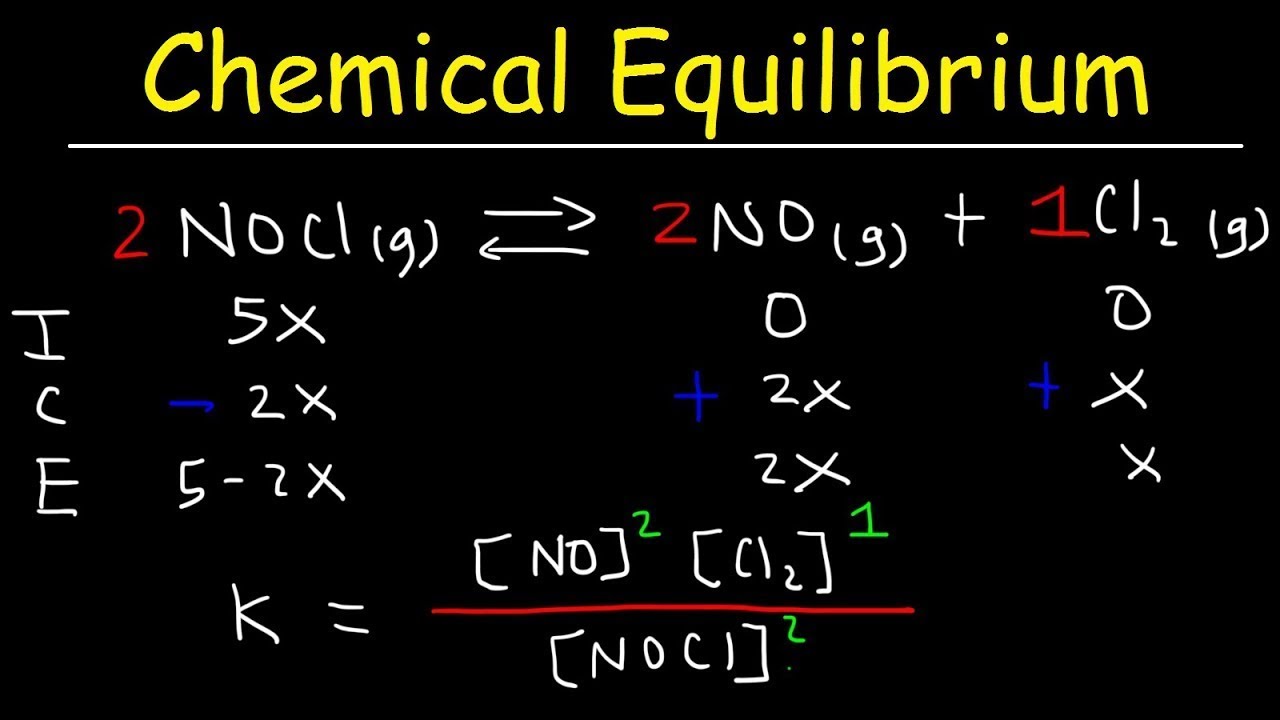
- 🧪 **Initial, Change, Equilibrium (ICE) Box**: A method to calculate concentrations at equilibrium by setting up initial amounts, changes, and equilibrium states.
- 📏 **Stoichiometry in Equilibria**: Stoichiometry is used to calculate the expected amounts of products in reversible reactions.
- 📉 **Reactant Depletion**: In the ICE box, reactants are represented with a negative change (-x) as they are consumed to form products.
- 📈 **Product Formation**: Products are represented with a positive change (+x) as they are formed from reactants.
- 📚 **Equilibrium Constant (Kc)**: Kc is the ratio of product concentrations to reactant concentrations, each raised to the power of their stoichiometric coefficients.
- 🔢 **Kc and Favorability**: A Kc much greater than one indicates a product-favored equilibrium, while a Kc much less than one indicates a reactant-favored equilibrium.
- 💧 **Exclusion of Solids and Pure Liquids**: Solids and pure liquids are not included in the Kc expression as their concentrations are constant.
- 🔮 **Reaction Quotient (Q)**: Q is calculated using non-equilibrium concentrations to predict the direction a reaction will proceed to reach equilibrium.
- ⚖️ **Equilibrium Concentrations**: Equilibrium concentrations are found by solving the Kc expression for x and substituting it back into the ICE box.
- 📐 **Solving for x**: In more complex equilibria, solving for x may require algebraic manipulation, such as taking square roots or using the quadratic equation.
Q & A
What is a reversible chemical reaction?
-A reversible chemical reaction is one where the reactants can create products, and then those products can react to form the original reactants. This means there is both a forward and reverse reaction occurring.
What is dynamic equilibrium in the context of chemical reactions?
-Dynamic equilibrium occurs when the rates of the forward and reverse reactions are the same, resulting in no net change in the concentrations of reactants and products, even though the reactions are still occurring.
How is stoichiometry used in discussing limiting reagents and product formation in unidirectional reactions?
-Stoichiometry is used to determine the amount of limiting reagents and the expected amount of products formed in unidirectional reactions, assuming all reactants are converted into products before the reaction stops.
What is the ICE (Initial, Change, Equilibrium) table used for in chemistry?
-The ICE table is a method used to calculate the concentrations of substances at equilibrium. It outlines the initial amounts, the changes that occur during the reaction, and the final equilibrium concentrations.
What does the equilibrium constant (Kc) represent?
-The equilibrium constant (Kc) represents the ratio of the concentrations of products to reactants, each raised to the power of their stoichiometric coefficients. It indicates whether a reaction favors the formation of products or reactants at equilibrium.
How is the equilibrium constant expression written?
-The equilibrium constant expression is written as the product of the concentrations of the products raised to their stoichiometric coefficients divided by the product of the concentrations of the reactants raised to their stoichiometric coefficients.
What does it mean if Kc is much greater than one?
-If Kc is much greater than one, it indicates that the reaction favors the formation of more products at equilibrium.
What does it mean if Kc is much less than one?
-If Kc is much less than one, it suggests that the reaction favors the formation of more reactants at equilibrium.
Why are solids and pure liquids not included in the equilibrium constant expression?
-Solids and pure liquids are not included in the equilibrium constant expression because their concentrations are constant and do not change during the reaction, making it unnecessary to account for them in the calculation.
What is the reaction quotient (Q) used for?
-The reaction quotient (Q) is used to predict the direction a reaction will proceed to reach equilibrium when given non-equilibrium concentrations of reactants and products. It is calculated using the same expression as Kc but with the current concentrations.
How can you determine if a system is at equilibrium using Kc and Q?
-If Kc is greater than Q, the reaction will proceed to make more products. If Kc is less than Q, it will make more reactants. If Kc equals Q, the system is already at equilibrium.
What is molarity and how is it used in calculating equilibrium concentrations?
-Molarity is the concentration of a substance expressed in moles per liter (mol/L). It is used in calculating equilibrium concentrations by providing the amount of solute in a given volume of solution.
Outlines
🔬 Understanding Chemical Equilibria
Professor Dave introduces the concept of chemical equilibria, explaining that some chemical reactions are reversible, with reactants and products interconverting. He describes dynamic equilibrium as a state where the rates of the forward and reverse reactions are equal, resulting in no apparent change despite ongoing chemical activity. The video covers the use of stoichiometry in unidirectional reactions and contrasts it with the more complex calculations required for equilibria. An 'ICE' table (Initial, Change, Equilibrium) is introduced as a method to calculate concentrations at equilibrium. The equilibrium constant, Kc, is explained as a way to determine whether a reaction favors products or reactants, with its calculation involving only gases and aqueous species, excluding solids and pure liquids. The concept of the reaction quotient, Q, is also discussed, which helps predict the direction a reaction will take to reach equilibrium.
🧮 Advanced Equilibrium Calculations
This paragraph delves into more complex equilibrium calculations, using stoichiometry to account for different changes in reactants and products. For every two moles of reactant consumed, one mole of each product is formed, which is reflected in the ICE table setup. The equilibrium concentrations are found by summing the initial and change values and plugging them into the equilibrium expression. The example provided shows how to solve for 'x', which represents the change in moles, and then use it to find all equilibrium concentrations. The video concludes with an encouragement to practice these calculations and an invitation to subscribe for more tutorials, as well as an offer to answer questions via email.
Mindmap
Keywords
💡Chemical Equilibria
💡Reversible Reactions
💡Dynamic Equilibrium
💡Stoichiometry
💡ICE Chart (Initial, Change, Equilibrium)
💡Equilibrium Constant (Kc)
💡Reaction Quotient (Q)
💡Limiting Reagents
💡Molarity
💡Stoichiometric Coefficients
💡Quadratic Equation
Highlights
Chemical reactions can be reversible, leading to a dynamic equilibrium where forward and reverse reactions occur at the same rate.
At dynamic equilibrium, there is a balance between reactants and products, even though chemical reactions continue to occur.
Stoichiometry is used to discuss limiting reagents and expected product amounts in unidirectional reactions.
Calculating concentrations at equilibrium for reversible reactions requires additional mathematical approaches.
An ICE (Initial, Change, Equilibrium) table is a method to track changes in reactant and product concentrations over time.
The equilibrium constant, Kc, is a ratio of product to reactant concentrations raised to their stoichiometric coefficients.
A high Kc value indicates a product-favored equilibrium, while a low Kc value indicates a reactant-favored equilibrium.
The equilibrium constant expression only includes gases and aqueous species; solids and pure liquids are excluded.
The reaction quotient, Q, is calculated using non-equilibrium concentrations to predict the direction a reaction will proceed.
If Kc is greater than Q, the reaction will proceed to produce more products; if Kc is less than Q, more reactants will form.
When Kc equals Q, the system is at equilibrium, indicating no net change in concentrations of reactants and products.
Different stoichiometric coefficients in a reaction require adjustments in the ICE table to accurately reflect changes in concentrations.
Equilibrium expressions can sometimes be simplified, such as taking the square root of both sides when dealing with squared terms.
In more complex cases, the quadratic equation may be necessary to solve for the equilibrium concentrations.
Molarity, measured in moles per liter, is used to express equilibrium concentrations in terms of substance amount and volume.
The ICE table and equilibrium constant calculations are essential tools for understanding and predicting chemical behavior in reversible reactions.
Professor Dave's tutorial provides a comprehensive understanding of chemical equilibria, including practical examples and problem-solving techniques.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: