Naming Cycloalkanes With Substituents, Cis & Trans, Bicyclo Alkane Nomenclature
TLDRThis video script offers an educational guide on naming cycloalkanes, a class of organic compounds. It begins with the basics of cyclopropane and cyclobutane, then progresses to more complex structures like cyclopentane and cyclohexane. The script teaches how to name compounds with substituents, such as ethyl and methyl groups, emphasizing the importance of alphabetical order and numbering. It also covers the nomenclature of bicyclic compounds, detailing how to identify bridgehead carbons and calculate the correct name based on the number of carbons in the rings and bridges. The explanation is methodical, ensuring viewers can follow the systematic approach to cycloalkane nomenclature.
Takeaways
- 🔡 The video focuses on naming cycloalkanes, which are cyclic hydrocarbons with carbon atoms arranged in a ring.
- 🔑 The prefix 'cyclo' is added to the name of the alkane corresponding to the number of carbons in the ring.
- 📝 Cyclopropane is named for a three-carbon ring, and cyclobutane, cyclopentane, cyclohexane, and cycloheptane follow the same naming convention for four, five, six, and seven carbon rings, respectively.
- 🎨 Viewers are encouraged to practice drawing cycloalkanes with more carbons, such as cycloheptane and cyclooctane, by starting with two lines and adding the appropriate number of carbons.
- 🔄 When a cycloalkane has substituents, such as an ethyl group, the name of the compound is formed by combining the substituent name with the cycloalkane name, like 'ethylcyclohexane'.
- 📐 For multiple substituents, they are listed in alphabetical order and numbered to indicate their position on the ring, as in '1-ethyl-2-methylcyclopentane'.
- 🔢 Numbers are separated by commas for multiple substituents on the same carbon, like '1,3-dimethylcyclobutane'.
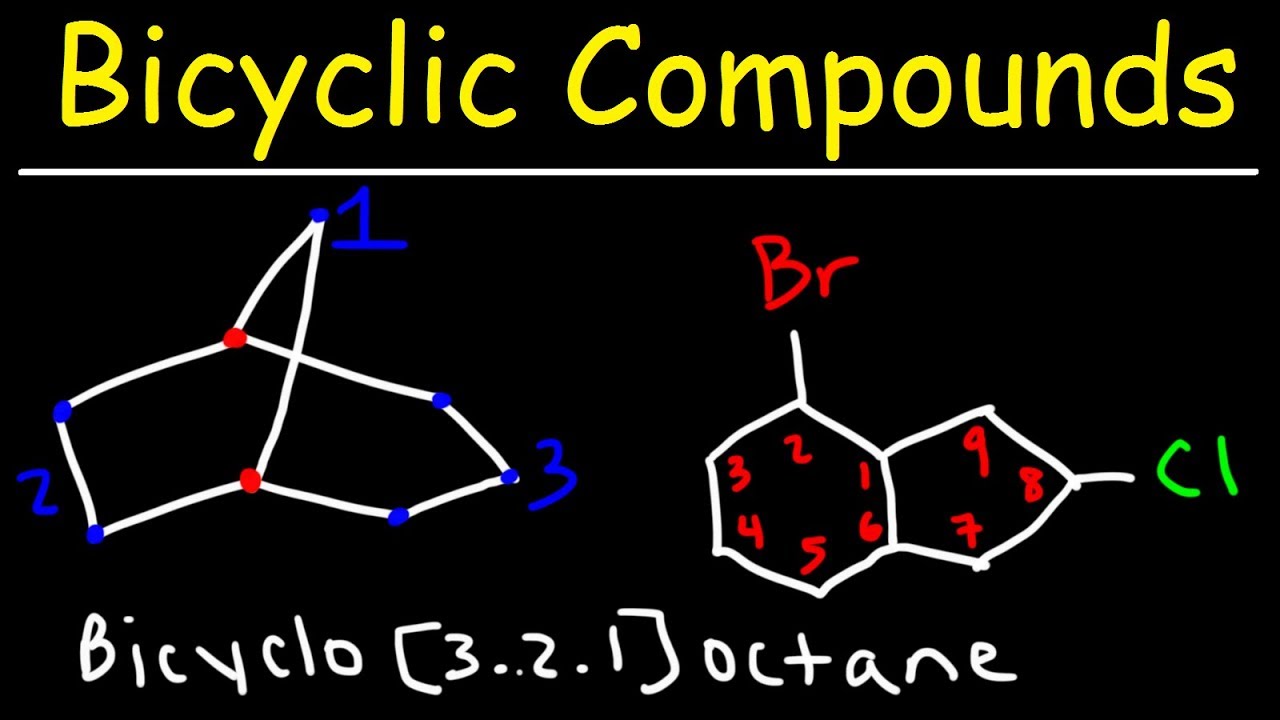
- 🌐 Bicyclic compounds are named by identifying the bridgehead carbons and counting the carbons between them in descending order, followed by the total number of carbons including the bridgehead carbons.
- 🔄 In bicyclic naming, the prefix 'bicyclo' is used, and the total number of carbons is the sum of the carbons between the bridgeheads plus the two bridgehead carbons.
- 🔍 Isomers like 'trans-1,2-dichlorocyclohexane' and 'cis-1,2-dibromocyclopentane' are named based on the position and orientation of the substituents relative to each other on the ring.
- 📖 The video emphasizes the importance of knowing the first 10 names of common alkanes as substituents to correctly name more complex cycloalkanes.
Q & A
What is the name of a three-carbon cycloalkane?
-A three-carbon cycloalkane is called cyclopropane.
How do you name a cycloalkane with four carbons?
-A four-carbon cycloalkane is named cyclobutane.
What is the name for a cycloalkane with five carbons in the ring?
-A cycloalkane with five carbons is called cyclopentane.
What is the process for drawing cyclohexane?
-To draw cyclohexane, start by putting two lines to represent the hexagon shape of the ring.
How do you draw cycloheptane?
-Cycloheptane can be drawn by starting with a seven-sided polygon to represent the ring.
What is the simplest way to draw cyclooctane?
-Cyclooctane is drawn as an eight-sided polygon representing the ring with eight carbons.
What is the name of a compound with a six-carbon ring and two carbons outside as a substituent?
-The compound is named as an ethyl cyclohexane, where 'ethyl' is the substituent with two carbons.
What is the correct order for naming multiple substituents on a cycloalkane?
-When naming multiple substituents, they should be listed in alphabetical order and numbered in increasing order.
How do you name a compound with a methyl and ethyl group on a cyclopentane ring?
-The compound is named as 1-ethyl-2-methylcyclopentane, with ethyl listed before methyl in alphabetical order and the numbers in increasing order.
What is the name for a compound with two methyl groups on a cyclobutane ring?
-The compound is named as 1,3-dimethylcyclobutane, indicating two methyl groups on carbons 1 and 3.
How do you name a compound with a cyclohexyl substituent on a heptane chain?
-The compound is named as heptylcyclohexane, with 'heptyl' indicating the seven-carbon chain and 'cyclohexane' as the substituent.
What is the name for a compound with a trans-1,2-dichlorocyclohexane structure?
-The compound is named as trans-1,2-dichlorocyclohexane, indicating the chlorine atoms are on opposite sides of the ring.
How do you name a compound with a cis-1,2-dibromocyclopentane structure?
-The compound is named as cis-1,2-dibromocyclopentane, indicating the bromine atoms are on the same side of the ring.
What is the naming convention for a bicyclic compound with bridgehead carbons?
-Bicyclic compounds are named by identifying the bridgehead carbons and the number of carbons between them in descending order, followed by the total number of carbons including the bridgehead carbons.
How do you name a bicyclic compound with two carbons between the bridgehead carbons on all sides?
-The compound is named as bicyclo[2.2.2]octane, indicating two carbons between each pair of bridgehead carbons and a total of eight carbons.
What is the name for a bicyclic compound with three carbons on the right side, two on the left, and one on top between the bridgehead carbons?
-The compound is named as bicyclo[3.2.1]octane, reflecting the arrangement of carbons and a total of eight carbons.
Outlines
🔬 Naming Cycloalkanes
This paragraph introduces the process of naming cycloalkanes, which are cyclic hydrocarbons. It explains that the naming convention involves adding the prefix 'cyclo' to the alkane name corresponding to the number of carbons in the ring. The video script covers cyclopropane, cyclobutane, cyclopentane, and cyclohexane, and instructs viewers to draw cycloheptane and cyclooctane. It also discusses the naming of cycloalkanes with substituents, such as ethyl and methyl groups, and how to name them in alphabetical order with increasing numbers when multiple substituents are present.
🧪 Complex Cycloalkane Nomenclature
This paragraph delves into the nomenclature of more complex cycloalkanes, including those with substituents and multiple rings. It explains how to identify the longest chain in a molecule and how to name substituents such as cyclohexyl. The script also covers the naming of cis and trans isomers, where the position of chlorine or bromine atoms on the ring affects the name. Additionally, it teaches how to name bicyclic compounds by identifying bridgehead carbons and the number of carbons between them, using the example of 'bicyclo[2.2.1]heptane' and 'bicyclo[3.2.1]octane'.
📚 Advanced Bicyclic Compound Nomenclature
The final paragraph focuses on the advanced nomenclature of bicyclic compounds, emphasizing the importance of identifying the bridgehead carbons and the carbons in between to determine the correct name. It provides an example of how to name a compound with two carbons on each side and two in the middle as 'bicyclo[2.2.2]octane'. This section reinforces the method of naming bicyclic compounds by counting the carbons in a descending order and adding the total number of carbons, including the bridgehead carbons, to arrive at the final name.
Mindmap
Keywords
💡Cycloalkanes
💡Cyclopropane
💡Cyclohexane
💡Substituents
💡Ethyl Group
💡Methyl Group
💡Bicyclic Compounds
💡Bridgehead Carbons
💡Isomers
💡Trans-Isomer
💡Cis-Isomer
Highlights
Introduction to naming cycloalkanes with an example of cyclopropane.
Explanation of the prefix 'cyclo' for ring structures in alkanes.
Naming of cycloalkanes with four carbons as cyclobutane.
Identification of common cycloalkanes: cyclopentane and cyclohexane.
Instructions to draw cyclohexane, cycloheptane, and cyclooctane.
Naming a six-carbon ring with two carbons outside as ethylcyclohexane.
Importance of knowing the first 10 names of common alkanes for substituents.
Alphabetical order rule for naming multiple substituents on cycloalkanes.
Numbering of substituents in increasing order for cycloalkane naming.
Naming a compound with a methyl and ethyl group on a cyclopentane ring.
Use of commas to separate numbers for multiple substituents on the same carbon.
Naming a compound with a cyclohexyl group as a substituent.
Differentiation between cis and trans isomers in cycloalkane naming.
Naming a compound with bromine and ethyl groups on a cyclohexane ring.
Bicyclic compound naming by identifying bridgehead carbons and carbon count.
Method for naming bicyclic compounds with varying numbers of carbons between bridgehead carbons.
Final example of naming a bicyclic compound as bicyclo[2.2.2]octane.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: