4.3 IUPAC Nomenclature of Bicyclic Compounds | Organic Chemistry
TLDRThe video script is an educational lesson focused on the nomenclature of bicyclic compounds in organic chemistry. The lesson is part of an organic chemistry playlist, aimed at making science understandable and enjoyable. It covers three types of bicyclic compounds: bridged, fused, and spiro bicyclics, each with unique naming conventions. The instructor, Chad, explains the process of identifying bridgehead carbons and numbering carbons in a specific order to name these compounds. The lesson also touches on the presence of substituents and how they affect the naming process. Additionally, Chad provides a brief overview of the prioritization of functional groups in organic chemistry nomenclature and encourages students to subscribe for weekly lessons throughout the 2020-21 school year.
Takeaways
- 🔬 **Bicyclic Compounds:** The lesson covers three variants: bridged, fused, and spiro bicyclics, each with specific rules for naming.
- 📝 **Naming Rules:** For bridged and fused bicyclics, a set of rules applies, while spiro bicyclics have a different set of naming rules.
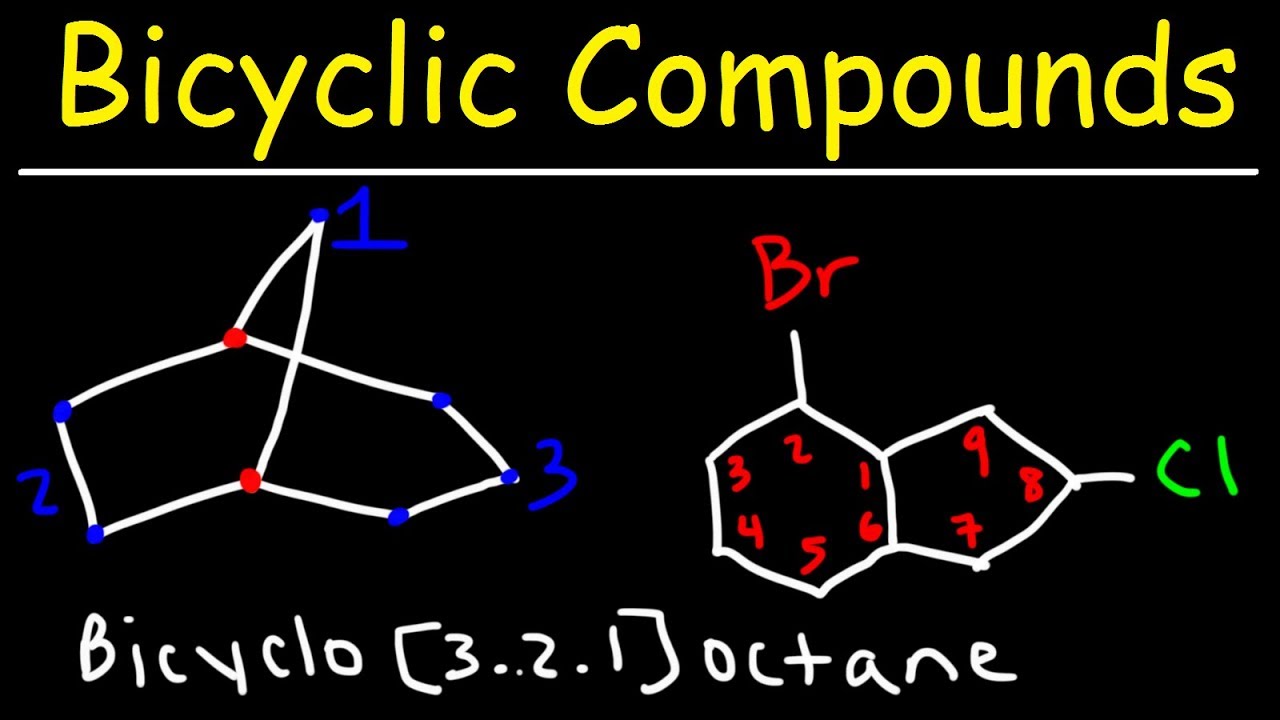
- 🔢 **Bridgehead Carbons:** In bridged bicyclics, the bridgehead carbons are part of every ring and are key to naming the compound.
- 📐 **Numbering Carbons:** The carbons between bridgehead carbons are counted and ordered from highest to lowest for naming, excluding the bridgehead carbons themselves.
- 🔄 **Substituents:** When present, substituents on bicyclic compounds are named by adding a prefix to the parent compound, with the position determined by numbering the carbons.
- 🎓 **Numbering Priority:** In bridged and fused bicyclics, one of the bridgehead carbons is assigned the number one, and numbering proceeds through the longest carbon chain first.
- 🔀 **Fused Bicyclics:** In fused bicyclics, the bridgehead carbons are directly bonded, and the third number in the name is always zero, reflecting this direct connection.
- ⚙️ **Spiro Bicyclics:** Spiro bicyclics have a spirocarbon that is a single shared carbon between two rings, and they are numbered from lowest to highest, starting from the spirocarbon.
- 📌 **Substituent Positioning:** In spiro bicyclics, the position of substituents is determined by numbering the smallest ring first and then proceeding to the larger one to achieve the lowest possible number for the substituent.
- 📚 **Functional Group Priorities:** As the course progresses, different functional groups will be encountered, each with a specific suffix or prefix depending on its priority in the molecule.
- 📈 **Study Guide Reference:** The provided study guide will be a useful reference for the ranking of functional groups and their naming conventions throughout the course.
Q & A
What are the three types of bicyclic compounds discussed in the lesson?
-The three types of bicyclic compounds discussed are bridged bicyclics, fused bicyclics, and spiro bicyclics.
What is the term used to describe the carbon atoms that are part of every ring in a bridged bicyclic compound?
-The term used is 'bridgehead carbons'.
How are the numbers in the name of a bridged bicyclic compound determined?
-The numbers are determined by counting the number of carbons between each pair of bridgehead carbons, not including the bridgeheads themselves, and arranging them in descending numerical order.
When naming a bicyclic compound with substituents, where is the numbering typically started from?
-The numbering typically starts from one of the bridgehead carbons, which is assigned the number one.
What is the difference between naming a bridged bicyclic compound and a fused bicyclic compound in terms of the numbers used in the name?
-For a fused bicyclic compound, the third number is always zero, indicating that the two bridgehead carbons are directly bonded to each other in one of the pathways.
How are the numbers in the name of a spiro bicyclic compound determined?
-The numbers are determined by counting the number of carbons in each pathway from the spirocarbon back to the spirocarbon, starting with the lowest number and moving to the highest.
What is the prefix used for naming spiro bicyclic compounds?
-The prefix used is 'spiro'.
When a substituent is present in a spiro bicyclic compound, how should the compound be numbered?
-The compound should be numbered by starting with the lower chain before the higher, with the carbon next to the spirocarbon being assigned the number one.
null
-null
What is the general ranking system for naming functional groups in organic chemistry?
-The general ranking system prioritizes functional groups, with the highest priority group being named as part of the parent chain, and additional groups being named as substituents with prefixes.
Why is it important to understand the nomenclature of bicyclic compounds?
-Understanding the nomenclature is important because it is a part of the curriculum for many students, and it helps in the systematic and clear communication of the structure of organic compounds.
What does the instructor suggest for students who find the lesson helpful?
-The instructor suggests that students who find the lesson helpful should consider giving a like and a share to support the channel.
Where can students find practice problems or study guides related to the lesson?
-Students can find practice problems or study guides related to the lesson on the instructor's website, chadsprep.com.
Outlines
🧪 Naming Bicyclic Compounds: An Introduction
This paragraph introduces the topic of naming bicyclic compounds, which are divided into three variants: bridged bicyclics, fused bicyclics, and spiro bicyclics. The speaker, Chad, explains that this lesson follows previous lessons on alkanes and complex substituents, and emphasizes the importance of learning these naming conventions before discussing conformational structures. Chad also introduces himself and his educational channel, encouraging viewers to subscribe for weekly updates. The paragraph outlines the special rules for naming bicyclic compounds, distinguishing between bridged and fused bicyclics, and introduces the concept of bridgehead carbons, which are key to the naming process.
🔢 Numbering and Naming Bridged Bicyclic Compounds
Chad explains how to identify bridgehead carbons in bridged bicyclic compounds and how to number the carbons between them to derive the name of the compound. The process involves counting the carbons between each pair of bridgehead carbons, excluding the bridgeheads themselves, and arranging these numbers in descending order. The paragraph also covers how to name substituents on these compounds, with an example of a methyl group attached to a bridged bicyclic compound. Chad demonstrates how to number the carbons to achieve the lowest possible number for the substituent, using the longest chain from one bridgehead to the other as the primary numbering sequence.
🔁 Numbering and Naming Spiro and Fused Bicyclic Compounds
The paragraph covers the naming and numbering of spiro and fused bicyclics. For fused bicyclics, where the bridgehead carbons are directly bonded, the third number in the name is always zero, and the numbering follows the same pattern as bridged bicyclics but includes the direct pathway between the bridgeheads. For spiro bicyclics, which share a single common carbon (spirocarbon), the numbering starts from the spirocarbon and moves outward, with the numbers arranged from lowest to highest, and the prefix 'spiro' is used instead of 'bicyclo'. Chad also discusses how to handle substituents in spiro compounds, emphasizing the importance of numbering the smallest chain first to achieve the lowest possible number for the substituent.
📚 Nomenclature Priorities and Future Reference
Chad discusses the importance of nomenclature in organic chemistry and provides a list of functional groups ranked by priority. This ranking determines whether a functional group will be named as a suffix on the parent chain or as a substituent with a prefix. The paragraph serves as a future reference for students as they encounter various functional groups throughout their studies. Chad also encourages students to like, share, and subscribe to his channel for support and mentions the availability of practice problems and study guides on his website.
Mindmap
Keywords
💡Bicyclic Compounds
💡Bridged Bicyclic
💡Fused Bicyclic
💡Spiro Bicyclic
💡Bridgehead Carbons
💡Naming Conventions
💡Substituents
💡Conformational Structures
💡Numbering System
💡Organic Chemistry
💡Functional Groups
Highlights
Introduction to naming bicyclic compounds in organic chemistry
Three variants of bicyclic compounds: bridged, fused by cyclics, and spiro bicyclics
Explanation of special rules for naming bicyclic compounds
Identification of bridgehead carbons in bicyclic structures
Method for naming bridged bicyclic compounds using numbers in descending order
Procedure for naming bicyclic compounds with substituents
Numbering system for identifying the position of substituents in bicyclic compounds
Differentiation between naming bridged and fused bicyclic compounds
Zero as the third number in the name of fused bicyclic compounds
Introduction to spiro bicyclics and the concept of spirocarbon
Naming spiro compounds by counting carbons from the spirocarbon to itself
Rule for numbering spiro compounds from lowest to highest
Addressing the presence of substituents in spiro bicyclics and their numbering
Explanation of the priority ranking system for naming functional groups in organic chemistry
Importance of understanding the nomenclature hierarchy for functional groups
Advice on the varying inclusion of bicyclic compound nomenclature in different curricula
Upcoming lessons on conformational structures of alkanes
Encouragement to subscribe for weekly updates on the organic chemistry playlist
Availability of practice problems and study guides on chadsprep.com
Transcripts
Browse More Related Video
Naming Bicyclic Compounds

Naming Cycloalkanes With Substituents, Cis & Trans, Bicyclo Alkane Nomenclature

4.2 Naming Complex Substituents | Organic Chemistry

6.5 Curved Arrow Pushing in Reaction Mechanisms | Organic Chemistry

Naming Organic Compounds 1

4.1 IUPAC Nomenclature of Alkanes and Cycloalkanes | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: