What Is Organic Chemistry?: Crash Course Organic Chemistry #1
TLDRIn this Crash Course Organic Chemistry episode, Deboki Chakravarti introduces the fascinating world of organic chemistry, which is centered around carbon atoms and their ability to form diverse compounds. The video explains that organic chemistry studies molecules with carbon atoms, which can create complex structures like chains, rings, and multi-ring systems. It delves into the history of organic chemistry, highlighting the discovery of urea by Friedrich Wöhler that marked a shift from solely obtaining chemicals from living organisms to synthesizing them. The episode also covers the importance of understanding molecular structures and reaction mechanisms, and introduces various ways to represent organic molecules, including Lewis structures, condensed structural formulas, and skeletal formulas. It concludes by emphasizing the relevance of organic chemistry in everyday life, from the compounds in our food to the materials in our technology.
Takeaways
- 🌟 Chemistry is the science of everything, from stars to computer hard drives and our bodies, all made of atoms bonded together in various ways.
- 📚 This course focuses on organic chemistry, which is the study of molecules containing carbon atoms that can form a vast array of compounds.
- 🔬 Carbon, with its four valence electrons, often forms chains or rings, leading to complex structures like steroids.
- 🌱 Organic chemistry was historically about compounds derived from living things, but the definition has expanded to include man-made polymers like plastics.
- 🔬 The term 'organic chemistry' was coined by Jöns Jacob Berzelius, who also contributed to modern chemical symbols and the discovery of several elements.
- 🧪 Friedrich Wöhler's synthesis of urea from an inorganic compound in 1828 marked the beginning of the modern organic chemical industry.
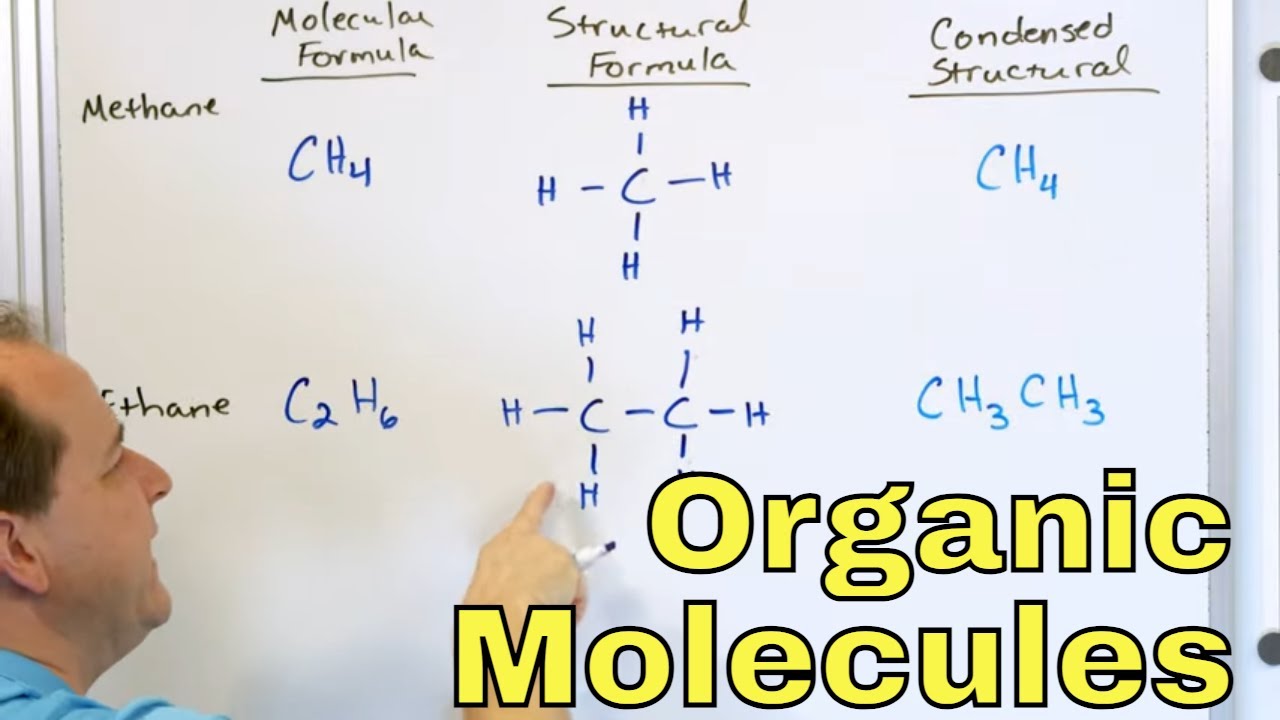
- 📝 Lewis structures, condensed structural formulas, and skeletal formulas are different ways to represent organic molecules, with each serving a specific purpose in chemistry.
- 🔍 Heteroatoms, atoms other than carbon and hydrogen in organic molecules, are important for understanding functional groups where most of the 'cool chemistry' occurs.
- 🌈 Organic compounds are responsible for the colors in many natural things and are used in various applications, from dyes to conductive polymers in electronics.
- 🚀 The development of conductive polymers in the 1970s was a significant breakthrough, leading to lighter electronic devices and full-color displays.
- 🌿 Organic compounds are prevalent in our daily lives, from the caffeine in our coffee to the molecules that enable vision, such as retinal.
- ✅ Upcoming episodes will delve into the nomenclature of organic molecules, building on the foundational concepts introduced in this episode.
Q & A
What is the main focus of organic chemistry?
-Organic chemistry is the study of molecules that have carbon atoms, which often bond to themselves to form various compounds.
What is the significance of carbon's four valence electrons in organic chemistry?
-Carbon's four valence electrons allow it to form four bonds, leading to the creation of complex and diverse organic compounds, such as chains and rings of carbons.
How did the term 'organic chemistry' originate?
-The term 'organic chemistry' was coined by the Swedish chemist Jöns Jacob Berzelius, who initially believed that organic compounds could only be derived from living things.
What was the breakthrough discovery by Friedrich Wöhler that challenged the traditional view of organic chemistry?
-Friedrich Wöhler discovered that urea, an organic compound, could be synthesized from an inorganic salt, ammonium cyanate, without the need for a living organism, marking the start of the modern organic chemical industry.
What are some common ways to represent organic molecules?
-Organic molecules can be represented through Lewis structures, condensed structural formulas, and skeletal formulas or line-angle formulas, each offering different levels of detail and simplicity.
Why is carbon considered the key atom in organic chemistry?
-Carbon is the key atom in organic chemistry because it is capable of forming a wide variety of stable covalent bonds, creating complex structures that are the basis of organic compounds.
How do heteroatoms differ from carbon atoms in organic compounds?
-Heteroatoms are atoms in organic molecules other than carbon and hydrogen. They are often highlighted in skeletal formulas and can include elements like nitrogen, oxygen, and sulfur, which contribute to the compound's reactivity.
What is the role of functional groups in organic chemistry?
-Functional groups are specific parts of an organic molecule that define its chemical properties and reactivity. They are key to understanding the structure and potential reactions of organic compounds.
What is the importance of the octet rule in drawing Lewis structures?
-The octet rule states that atoms tend to form enough bonds to have eight electrons in their valence shell, which is particularly important for carbon atoms in organic chemistry to ensure stability.
How did ancient civilizations use urea in the textile industry?
-Ancient civilizations used urea, a nitrogen-containing compound found in urine, as a dye mordant to enhance the color and longevity of dyes on fabrics, particularly for indigo dye.
What is the modern definition of organic chemistry?
-Modern organic chemistry is defined as the study of the structure, properties, composition, reactions, and preparation of carbon-containing compounds, which includes both naturally occurring and synthetic materials.
How do organic compounds contribute to everyday life?
-Organic compounds are ubiquitous in everyday life, from the caffeine in coffee that helps us wake up, to the dyes in our clothing, the polymers in electronic devices, and the molecules in our eyes that enable vision.
Outlines
🌟 Introduction to Organic Chemistry
Deboki Chakravarti introduces the viewer to the world of organic chemistry, emphasizing its relevance to everything around us, from stars to computer hard drives. The focus is on molecules containing carbon atoms, which due to carbon's four valence electrons, can form a variety of complex compounds. The video outlines the historical development of organic chemistry, starting from the mid-1800s, and touches on the evolution of the term 'organic' from compounds derived from living things to the modern definition that includes all carbon-containing compounds. The importance of understanding molecular structures and reaction mechanisms is highlighted, and the video ends with a teaser for upcoming topics, including the naming of organic molecules.
📚 Understanding Organic Molecules and Their Structures
This segment delves into the representation of organic molecules, starting with Lewis structures that depict the connections between atoms and their bonds. The example of propane (C3H8) is used to illustrate how to draw a Lewis structure. The video then simplifies this process by introducing condensed structural formulas and skeletal formulas, which are more efficient for chemists. It also introduces the concept of heteroatoms and functional groups, which are crucial for understanding the chemistry of organic compounds. The practical applications of organic compounds in everyday life, such as in the food we eat, the clothes we wear, and the technology we use, are discussed. The video concludes with a look forward to the next episode, which will cover the nomenclature of organic molecules.
Mindmap
Keywords
💡Organic Chemistry
💡Carbon
💡Valence Electrons
💡Lewis Structure
💡Condensed Structural Formula
💡Skeletal Formula
💡Heteroatoms
💡Functional Groups
💡Urea
💡Polymers
💡Nomenclature
Highlights
Chemistry is the science of everything, from stars to computer hard drives to our bodies, all composed of atoms bonded and reacting with each other.
Organic chemistry focuses on molecules containing carbon atoms, which can form a vast array of compounds due to carbon's ability to bond with itself.
The term 'organic chemistry' was coined by Swedish chemist Jöns Jacob Berzelius and initially referred to compounds extracted from living organisms.
German chemist Friedrich Wöhler's synthesis of urea from an inorganic compound in 1828 marked the beginning of the modern organic chemical industry.
Organic chemistry today includes the study of both natural and man-made carbon-containing compounds, such as plastics.
Carbon's predictable behavior, such as forming four bonds, is central to understanding organic chemistry.
Lewis structures are used to illustrate the connections and bonds between atoms in organic molecules.
Condensed structural formulas and skeletal formulas are simplifications used to represent organic molecules more efficiently.
Organic molecules can be simplified to skeletal formulas where carbon atoms are implied at the bends or ends of lines, and hydrogen atoms are not shown.
Functional groups, which are parts of organic structures containing non-carbon atoms or multiple bonds, are central to the chemistry of these compounds.
Organic compounds are prevalent in everyday life, from the caffeine in coffee to the dyes in our clothes and the polymers in our electronics.
The discovery of how to make plastics conduct electricity in the 1970s was a significant breakthrough, leading to lighter electronic devices.
Natural organic compounds, such as betanin in beets, are responsible for the colors in many of the foods and materials we interact with daily.
Ancient civilizations used urea, a component of urine, as a dye additive, highlighting the historical use of organic compounds.
The course will cover the logic behind molecular structures and chemical reaction mechanisms, treating complex problems like puzzles.
The birth of modern organic chemistry is explored, starting from the mid-1800s with the extraction of medicinal chemicals from plants.
The importance of understanding and being able to draw different representations of organic compounds, from Lewis structures to skeletal formulas, is emphasized.
The course will delve into the nomenclature of organic molecules in future episodes, building on the foundational concepts introduced.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: