2.1 Condensed Structures | Organic Chemistry
TLDRThe video script focuses on the topic of molecular representations in organic chemistry, specifically discussing condensed structures and their role in illustrating organic molecules. It explains how to convert these condensed structures into Lewis structures, emphasizing the importance of understanding the bonding patterns of different atoms like carbon, hydrogen, and oxygen. The script also touches on the use of parentheses in condensed structures to represent repeating units or multi-atom branches. It delves into the concept of pi bonds, explaining how they are used to satisfy the bonding requirements of atoms that are part of a chain or a branch. Additionally, the script introduces functional groups and their significance in determining the chemical reactivity of molecules. The lesson is part of a new organic chemistry playlist released weekly throughout the 2020-21 school year, encouraging viewers to subscribe for updates.
Takeaways
- 📚 **Condensed Structures**: A way to efficiently represent organic molecules, focusing on carbon chains and their attached hydrogens.
- 🔍 **Lewis Structures**: Used to depict the bonding of atoms in a molecule, showing how carbon atoms typically aim for four bonds and hydrogens for one.
- 📈 **Efficiency in Drawing**: Organic chemists use condensed structures to minimize the amount of drawing needed, often employing parentheses for clarity and efficiency.
- 🔄 **Repeating Units**: Parentheses are used to denote repeating units, specifically methylene groups (CH2), in a carbon chain for simplicity.
- ➡️ **Branches in Structures**: Parentheses also enclose multi-atom branches off the main carbon chain, distinguishing them from single-atom branches like halogens.
- 🏎️ **Multiple Identical Branches**: A subscript number within parentheses indicates multiple identical branches off a carbon, further simplifying the structure.
- 🔗 **Pi Bonds**: When adjacent atoms lack a full octet, pi bonds (double or triple) are introduced to satisfy their bonding needs.
- 🚫 **Oxygen in Chains**: Oxygen typically forms two bonds and can be situated in the middle of a chain or as a branch, which can sometimes be ambiguous in condensed structures.
- ⚠️ **Deduction of Structure**: The need for additional bonds in certain atoms can lead to the deduction that an oxygen atom is actually a branch, not part of the main chain.
- 🔬 **Functional Groups**: The script introduces functional groups, such as ketones and carboxylic acids, which are characterized by specific bonding patterns and are central to organic chemistry.
- 📅 **Upcoming Lessons**: The speaker mentions a series of lessons planned for the 2020-21 school year, covering topics from molecular representations to resonance.
Q & A
What is the main topic of the lesson in the transcript?
-The main topic of the lesson is molecular representations, specifically focusing on how to draw organic molecules in addition to Lewis structures, condensed structures, bond line structures, functional groups, and resonance.
What is a condensed structure in organic chemistry?
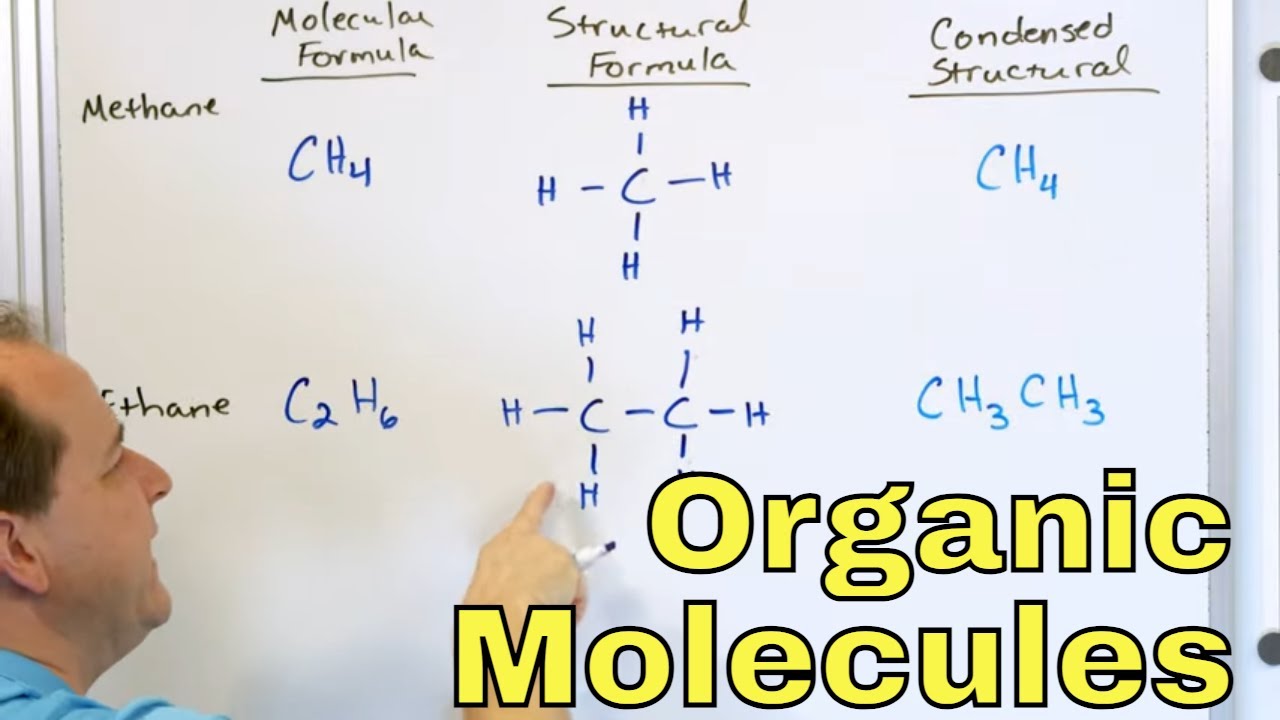
-A condensed structure is a simplified way of representing organic molecules where carbon atoms are listed with the number of hydrogen atoms attached to them, and carbon chains form the backbone of most molecules.
How are parentheses used in condensed structures?
-Parentheses are used in three different ways in condensed structures: 1) to denote repeating CH2 (methylene) groups, 2) to indicate multi-atom branches coming off the main carbon chain, and 3) to show multiple identical multi-atom branches attached to the same carbon.
What is the typical number of bonds a neutral carbon atom forms?
-A neutral carbon atom typically forms four bonds.
How do you determine if a double or triple bond is present in a condensed structure?
-You can determine the presence of a double or triple bond by looking for adjacent atoms that both need more electrons to complete their octet. If two atoms are adjacent and both lack one bond, a double bond is typically drawn between them. If they both lack two bonds, a triple bond is drawn.
What is a functional group?
-A functional group is a specific group of atoms within a molecule that is responsible for the characteristic chemical reactions of that molecule. Examples given in the transcript include ketones and carboxylic acids.
How does the presence of oxygen in a condensed structure affect the bonding?
-Neutral oxygen typically forms two bonds, which allows it to be in the middle of a chain if it can branch in two directions. If oxygen is part of a branch, it is not enclosed in parentheses because it is a single atom.
What is a bond line structure?
-A bond line structure, also known as a skeletal formula, is a type of chemical representation where only the bonds between atoms are drawn, and the atoms themselves are implied or represented by the endpoints of the lines.
What is resonance in chemistry?
-Resonance in chemistry refers to a way of describing the delocalization of electrons within a molecule, where a single Lewis structure cannot fully represent the bonding situation. It involves the phenomenon where the true structure of the molecule is an average of two or more contributing structures.
How often will the lessons be released in the new organic chemistry playlist?
-The lessons will be released weekly throughout the 2020-21 school year.
Why do organic chemists use condensed structures?
-Organic chemists use condensed structures to efficiently represent complex organic molecules without having to draw every atom and bond, thus saving time and space.
What is the significance of pi bonds in molecular structures?
-Pi bonds are significant as they represent a type of covalent bond where the shared electrons are located above and below the plane of the atoms involved, as seen in double and triple bonds, contributing to the molecule's overall shape and reactivity.
Outlines
📚 Introduction to Condensed Structures in Organic Chemistry
This paragraph introduces the topic of condensed structures as part of the broader subject of molecular representations and resonance in organic chemistry. The focus is on learning to draw organic molecules, including the use of Lewis structures and bond-line structures. The script explains how to represent carbon chains and hydrogens in a condensed structure, emphasizing the typical four-bond configuration of a neutral carbon atom. It also introduces the concept of functional groups and resonance, and encourages viewers to subscribe to the channel for weekly updates throughout the 2020-21 school year.
🔍 Using Parentheses in Condensed Structures
The second paragraph delves into the use of parentheses in condensed structures, highlighting three distinct scenarios. The first use is for repeating CH2 groups, known as methylene groups, to simplify the representation of long carbon chains. The second use is for multi-atom branches off the main carbon chain, which necessitates the use of parentheses to clarify the structure. The third case, a special instance of the second, involves showing multiple identical branches attached to the same carbon atom, indicated by a subscript. The paragraph also discusses the representation of pi bonds when adjacent atoms lack a filled octet, suggesting double or triple bonds to satisfy their bonding needs.
🧪 Deciphering Oxygen's Role in Condensed Structures
The final paragraph addresses the portrayal of oxygen within condensed structures, noting that oxygen typically forms two bonds and can be situated either in the middle of a chain or as a branch. The paragraph illustrates how to determine whether oxygen is part of the main chain or a branch by examining the bonding requirements of adjacent atoms. It also provides examples of how oxygen's positioning can affect the overall structure, leading to the formation of functional groups such as ketones and carboxylic acids. The summary underscores the importance of understanding the bonding needs of atoms to accurately represent organic molecules in condensed form.
Mindmap
Keywords
💡Condensed Structures
💡Lewis Structures
💡Carbon Chain
💡Methylene Groups
💡Functional Groups
💡Resonance
💡Bond Line Structures
💡Octet Rule
💡Pi Bonds
💡Formal Charge
💡Ketone
💡Carboxylic Acid
Highlights
Introduction to condensed structures and their importance in organic chemistry.
Explanation of how to draw organic molecules using condensed structures and Lewis structures.
Use of parentheses in three different ways to simplify condensed structures.
Methylene groups (CH2) are used to represent repeating units in a carbon chain.
How to represent multi-atom branches coming off the main carbon chain using parentheses.
Indicating multiple identical branches using a subscript with parentheses.
The role of pi bonds in structures where adjacent atoms need additional electrons.
Adding double or triple bonds between carbon atoms to satisfy their valency.
Common occurrence of oxygen in organic molecules and its typical bonding behavior.
Determining whether oxygen is part of the main chain or a branch in a molecule.
The default assumption that oxygen is in the middle of a carbon chain unless evidence suggests otherwise.
Identifying functional groups such as ketones and carboxylic acids in molecular structures.
The significance of formal charges in altering the typical number of bonds an atom will form.
Efficient representation of organic molecules through the use of condensed structures.
The process of converting a condensed structure into a Lewis structure while keeping in mind the valency of different atoms.
How to recognize and represent branches with multiple atoms in a condensed structure.
The use of pi bonds to achieve a stable octet for atoms that are short of electrons.
The importance of understanding the bonding patterns of halogens and their typical single bond formation.
Transcripts
Browse More Related Video

16.1 Hydrocarbons | High School Chemistry

2.2 Drawing Line Angle Structures (aka Bond Line Structures) | Organic Chemistry

What Is Organic Chemistry?: Crash Course Organic Chemistry #1

1.3 Valence Bond Theory and Hybridization | Organic Chemistry
Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]

Bond Line Formulas, Lewis Structures, Kekule & Condensed Structures Molecular Representations
5.0 / 5 (0 votes)
Thanks for rating: