16.1 Hydrocarbons | High School Chemistry
TLDRThis video script introduces organic chemistry, focusing on hydrocarbons—compounds consisting solely of hydrogen and carbon. It explains the significance of carbon in life and its unique bonding capabilities, leading to the complexity of organic molecules. The lesson categorizes hydrocarbons into alkanes, alkenes, alkynes, and aromatics, highlighting their structures and reactivity. It also covers molecular representations, including structural, condensed, and molecular formulas, with examples to illustrate different isomers. The script is part of a high school chemistry series, aiming to educate and simplify the foundational concepts of organic chemistry.
Takeaways
- 🧪 Organic chemistry is the study of carbon-based compounds, which are fundamental to life and can also be found outside of living organisms.
- 🌟 Hydrocarbons, compounds consisting solely of hydrogen and carbon, are categorized into four classes: alkanes, alkenes, alkynes, and aromatics.
- 🔍 Carbon's ability to form four bonds due to its four valence electrons, and its small size allowing for double and triple bonds, contributes to the complexity and variety of organic molecules.
- 📐 The hybridization of carbon atoms (sp3, sp2, sp) affects the molecular geometry, which can be tetrahedral, trigonal planar, or linear.
- 🔥 Alkanes, characterized by single carbon-carbon bonds, are relatively unreactive but are the main components of gasoline and undergo combustion.
- ⚡ Alkenes and alkynes contain carbon-carbon double and triple bonds, respectively, which introduce reactivity, especially in addition reactions.
- 💫 Aromatic compounds like benzene have a stable, delocalized pi electron system that makes them less reactive than alkenes and alkynes.
- 🔑 The presence of pi bonds in hydrocarbons generally indicates unsaturation, meaning they do not have the maximum possible number of hydrogen atoms.
- 📝 Molecular representations in organic chemistry include Lewis dot structures, line angle structures, condensed formulas, and molecular formulas.
- 🌐 Condensed formulas simplify the depiction of hydrocarbons by abbreviating repeating units or indicating branches with parentheses.
- 📚 The script is part of a high school chemistry playlist aimed at educating students on organic chemistry concepts throughout the school year.
Q & A
What is the main focus of the first lesson in the chapter on organic chemistry?
-The main focus of the first lesson is to introduce the subject of organic chemistry, starting with the definition and moving on to discuss hydrocarbons, which are compounds made only of hydrogen and carbon.
Why are organic compounds important in the context of life?
-Organic compounds are important because they are carbon-based compounds that form the basis of life. All life is based on these compounds, which include elements like carbon, hydrogen, oxygen, and nitrogen, with carbon being the central element due to its unique bonding capabilities.
What makes carbon unique as a building block for life?
-Carbon is unique because of its ability to form four bonds due to its four valence electrons, its small size that allows for double and triple bonds, and the flexibility in the structures it can form, contributing to the complexity of organic molecules.
Why is silicon not considered a potential building block for life as carbon is?
-Silicon, despite having four valence electrons like carbon, is not a potential building block for life because it is larger and cannot form pi bonds as effectively. This limits the variety of structures it can form, unlike carbon.
What are the four classes of hydrocarbons mentioned in the script?
-The four classes of hydrocarbons mentioned are alkanes, alkenes, alkynes, and aromatics.
How are alkanes described in terms of their carbon-carbon bonds?
-Alkanes are described as having only single carbon-carbon bonds, with no double or triple bonds present.
What is the characteristic feature of alkenes that makes them more reactive than alkanes?
-Alkenes are characterized by the presence of a carbon-carbon double bond, which includes a pi bond, making them more reactive than alkanes.
What is the significance of the term 'saturated' in organic chemistry?
-In organic chemistry, 'saturated' refers to compounds that have the maximum number of hydrogen atoms possible, typically found in straight chain or branched alkanes without any rings or pi bonds.
How does the presence of a pi bond affect the reactivity of hydrocarbons?
-The presence of a pi bond in hydrocarbons, as seen in alkenes and alkynes, increases their reactivity, making them more prone to addition reactions compared to saturated hydrocarbons.
What is the difference between a structural formula and a condensed formula in organic chemistry?
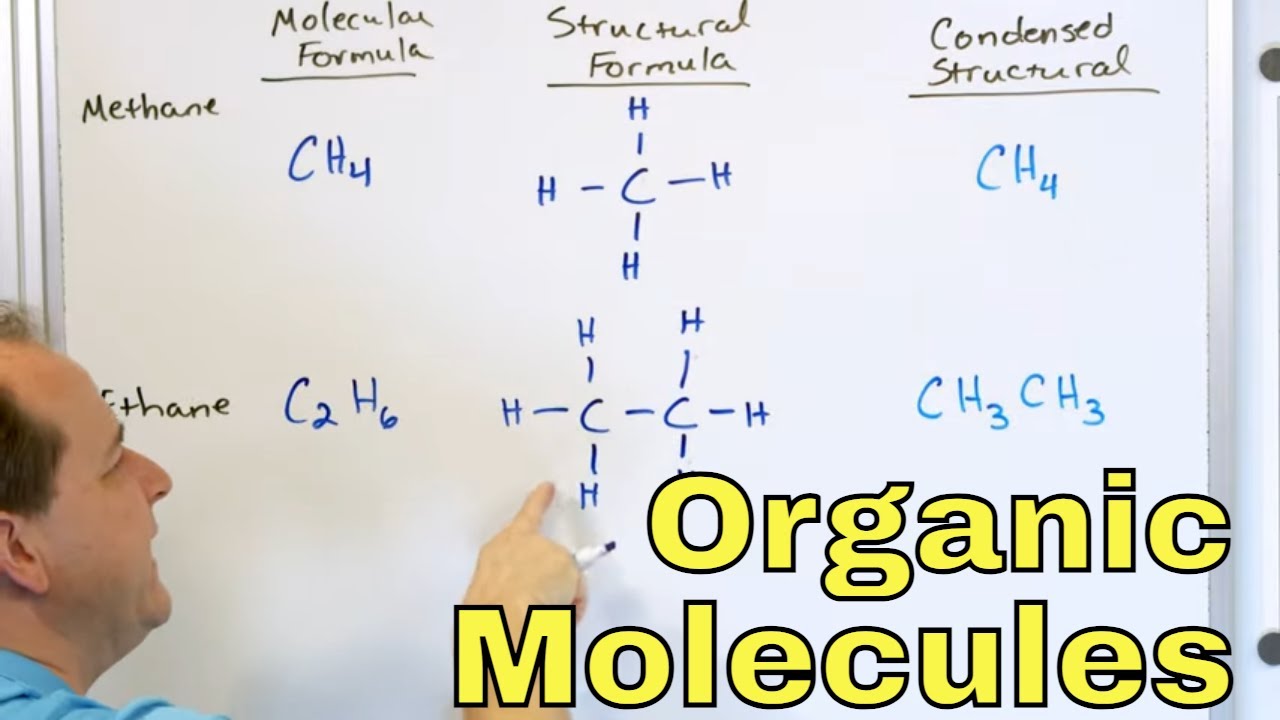
-A structural formula is a detailed representation of a molecule showing all atoms and bonds, while a condensed formula simplifies the representation by omitting some details and using abbreviations, making it easier to draw complex molecules.
What is the molecular formula and how does it differ from a structural or condensed formula?
-The molecular formula is a representation that states the total number of each type of atom in a molecule. It differs from a structural or condensed formula in that it does not provide information about the arrangement or connectivity of atoms, only their counts.
Outlines
🧪 Introduction to Organic Chemistry and Hydrocarbons
This paragraph introduces the topic of organic chemistry, emphasizing its focus on carbon-based compounds which are fundamental to life. It clarifies the common misconception about the term 'organic' in relation to food and highlights the importance of carbon, hydrogen, oxygen, and nitrogen in organic compounds. The unique ability of carbon to form diverse molecular structures due to its four valence electrons and small size is discussed, which is crucial for the complexity and variety seen in organic molecules. The paragraph also introduces hydrocarbons, compounds consisting solely of carbon and hydrogen, and outlines the four classes of hydrocarbons: alkanes, alkenes, alkynes, and aromatics, setting the stage for further exploration in the lesson.
🔍 Classes of Hydrocarbons and Their Properties
This paragraph delves into the different classes of hydrocarbons, starting with alkanes, which are saturated hydrocarbons with only single bonds between carbon atoms. It explains the concepts of straight-chain, branched, and cycloalkanes, and how the presence of rings or multiple bonds reduces the maximum number of hydrogen atoms, classifying them as unsaturated. The paragraph then contrasts alkanes' relative inactivity with the increased reactivity of alkenes and alkynes due to their double and triple bonds, respectively. It also introduces aromatic compounds, particularly benzene, which are highly stable due to the delocalization of pi electrons in their conjugated ring structures. The summary also touches on the general reactivity trends of these hydrocarbons, with a focus on their typical reactions and stability.
📚 Molecular Representations in Organic Chemistry
The final paragraph discusses the various ways to represent molecular structures in organic chemistry, moving from the detailed Lewis dot structure to more simplified forms like the condensed formula. It explains the rationale behind these simplifications, which stem from the repetitive nature of drawing large organic molecules with many carbon and hydrogen atoms. The paragraph outlines the process of creating a condensed formula, which involves noting the number of hydrogen atoms attached to each carbon in the chain and indicating branches or repeating units with parentheses. The molecular formula, which lists the total count of each type of atom in a molecule, is also explained, highlighting its role in distinguishing between different structural isomers. The paragraph concludes with an example of how to represent a branched alkane using a condensed formula and its corresponding molecular formula.
Mindmap
Keywords
💡Organic Chemistry
💡Hydrocarbons
💡Alkanes
💡Alkenes
💡Alkynes
💡Aromatics
💡Saturated Hydrocarbons
💡Unsaturated Hydrocarbons
💡Lewis Dot Structure
💡Condensed Formula
💡Molecular Formula
Highlights
Introduction to the subject of organic chemistry, emphasizing its importance in the study of carbon-based compounds.
Explanation of the term 'organic' in chemistry, distinguishing it from its popular usage related to food and natural products.
Overview of hydrocarbons as compounds consisting solely of hydrogen and carbon, categorized into four classes: alkanes, alkenes, alkynes, and aromatics.
Discussion on the uniqueness of carbon as a building block of life due to its ability to form diverse structures with its four valence electrons.
Description of carbon's structural flexibility, including sp3, sp2, and sp hybridization, and its implications for molecular geometry.
Comparison of carbon with other elements like nitrogen, oxygen, and silicon, highlighting why carbon is essential for the complexity of life.
Introduction to alkanes, emphasizing their single carbon-carbon bonds and their classification into straight-chain, branched, and cycloalkanes.
Explanation of saturated hydrocarbons, including the concept of maximum hydrogen atoms and the impact of rings and pi bonds on saturation.
Discussion on the reactivity of alkanes, noting their relative unreactivity outside of combustion reactions.
Introduction to alkenes, focusing on their carbon-carbon double bonds and their tendency for addition reactions due to pi bonds.
Description of alkynes, characterized by carbon-carbon triple bonds, and their associated reactivity.
Explanation of aromatic compounds, particularly benzene, and their unique stability due to delocalized pi electrons.
Differentiation between saturated and unsaturated fats in the context of hydrocarbons, relating to the presence of pi bonds or rings.
Overview of molecular representations in organic chemistry, including structural formulas, condensed formulas, and molecular formulas.
Illustration of how to represent branched alkanes using condensed formulas, distinguishing between branches and repeating units.
Importance of molecular formulas in identifying the number of carbon and hydrogen atoms in a hydrocarbon.
Invitation for viewers to like, share, and subscribe for notifications on new lessons and playlists.
Promotion of a premium course for high school chemistry students, offering study guides and practice problems.
Transcripts
Browse More Related Video

Introduction to Organic Chemistry
Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]

2.1 Condensed Structures | Organic Chemistry

Organic Chemistry Introduction Part 1

What Is Organic Chemistry?: Crash Course Organic Chemistry #1

Organic Chemistry Drawing Structures - Bond Line, Skeletal, and Condensed Structural Formulas
5.0 / 5 (0 votes)
Thanks for rating: