3D Structure and Bonding: Crash Course Organic Chemistry #4
TLDRThis Crash Course Organic Chemistry episode dives into the 3D shapes of organic molecules, which are crucial for understanding their interactions and functions. Deboki Chakravarti explains the importance of visualizing molecules in three dimensions, using analogies like imagining a cat in 2D versus 3D. The video covers the evolution of theories from Lewis structures to Valence Shell Electron Pair Repulsion (VSEPR) theory, and introduces the concept of orbital hybridization, which combines s and p orbitals to form hybrid orbitals that predict molecular geometries. Examples like methane, ethene, and ethyne are used to illustrate how these theories apply to different types of bonds. The episode also touches on the historical discovery of DNA's structure and the role of isomers in organic chemistry, highlighting constitutional and geometric isomers. It concludes by emphasizing the significance of molecular shape in chemical reactions and the broader implications for life and the universe.
Takeaways
- 🧬 Understanding the 3D shapes of organic molecules is crucial for grasping how their structure affects their function in biological systems.
- 📐 VSEPR theory helps predict the 3D geometry of molecules based on the repulsion between electron pairs around a central atom.
- 🔬 There are three primary electron-pair geometries used in organic chemistry: linear, trigonal planar, and tetrahedral.
- 🧠 Molecular shapes are described by how atoms relate to each other, often ignoring lone pairs, which are important for the 3D shape.
- 🌐 Orbital hybridization is a concept that combines s and p orbitals to form hybrid orbitals, which better explain the 3D shapes of molecules.
- ⚛️ Valence bond theory, which involves the overlap of atomic orbitals, is fundamental to understanding how chemical bonds form the structure of molecules.
- 💧 The structure of DNA was elucidated with the help of valence bond theory and the concept of hybrid orbitals, leading to significant advancements in genetics.
- 🔑 Isomers are molecules with the same molecular formula but different arrangements of atoms, and they can be constitutional or geometric.
- 🛤️ Constitutional isomers differ in the connectivity of their atoms, whereas geometric isomers differ in the spatial arrangement of their bonds.
- 🔬 The discovery of DNA's structure was a result of challenging and correcting existing scientific models, emphasizing the importance of critical thinking in science.
- 🔬 Organic chemistry focuses on carbon compounds, but the principles of electron-pair geometries and hybrid orbitals apply to other elements as well, influencing their bonding and reactivity.
Q & A
What is the significance of understanding the 3D shapes of organic molecules?
-Understanding the 3D shapes of organic molecules is crucial because it helps us comprehend how the structure of a molecule affects its function. This knowledge is essential for predicting how different compounds will interact, which is fundamental in fields like biochemistry and pharmaceuticals.
What are the five generally accepted VSEPR electron-pair geometries?
-The five generally accepted VSEPR electron-pair geometries are linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral. However, in organic chemistry, three of these geometries are predominantly used: linear, trigonal planar, and tetrahedral.
How does the Valence Shell Electron Pair Repulsion (VSEPR) theory explain the 3D shape of a molecule?
-VSEPR theory explains that the 3D shape of a molecule is determined by the repulsion between the electron pairs in the valence shell of the central atom, which includes both the lone pairs of electrons and the atoms it is bonded to.
What is the role of hybridization in molecular geometry?
-Hybridization is the concept where atomic orbitals combine to form new hybrid orbitals that are suitable for bonding. This process helps to explain the three-dimensional shapes of molecules, as different hybrid orbitals (sp, sp2, sp3) result in different geometries (linear, trigonal planar, tetrahedral).
How does the structure of DNA relate to the concept of hybridization?
-The structure of DNA involves the hybridization of atomic orbitals, particularly in the formation of the carbonyl groups that are part of the nucleotide bases. The correct hybridization of oxygen atoms as sp2 allowed the nitrogenous bases to form hydrogen bonds, which was a key to understanding the double helix structure of DNA.
What is the difference between constitutional isomers and geometric isomers?
-Constitutional isomers, also known as structural isomers, have the same molecular formula but different arrangements of atoms. Geometric isomers, on the other hand, have the same molecular formula and atom-to-atom connections but differ in the spatial arrangement of the atoms or groups around a bond, particularly around a double bond where free rotation is not possible.
How does the concept of isomers relate to the structure of octane and iso-octane?
-Octane and iso-octane are constitutional isomers because they have the same molecular formula (C8H18) but different structural arrangements. Octane has a linear structure, while iso-octane has a branched structure, which is why it is also known as 2,2,4-trimethylpentane.
What is the significance of sigma and pi bonds in the context of valence bond theory?
-Sigma and pi bonds are types of covalent bonds that result from the overlap of atomic orbitals. Sigma bonds are formed by the direct, head-on overlap of orbitals, while pi bonds result from the sideways overlap. These bonds are central to understanding the structure and reactivity of molecules, particularly in molecules with double or triple bonds.
How does the shape of a methane molecule relate to its hybridization?
-The shape of a methane molecule is tetrahedral, which is a result of the carbon atom's sp3 hybridization. The carbon atom forms four sp3 hybrid orbitals, each of which overlaps with a 1s orbital from a hydrogen atom, resulting in four sigma bonds that give methane its tetrahedral geometry.
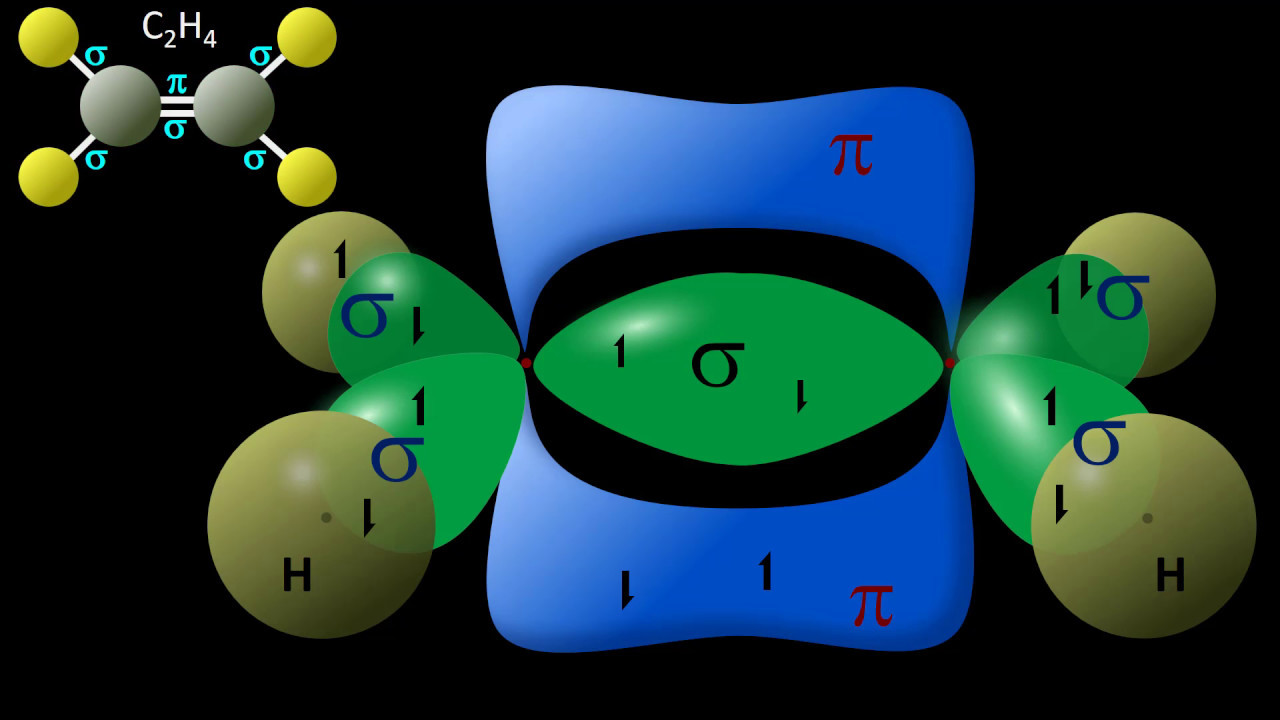
What is the role of the pi bond in the structure of ethene?
-In ethene (C2H4), each carbon atom is sp2 hybridized, forming three sigma bonds with other atoms. The pi bond in ethene is formed by the sideways overlap of the remaining p orbitals on each carbon atom, which adds stability and contributes to the molecule's trigonal planar geometry around each carbon.
Why is the concept of free rotation important when discussing geometric isomers?
-Free rotation is important because it determines whether a molecule can have geometric isomers. If a molecule has free rotation around a single bond, it won't have geometric isomers. However, if rotation is restricted, as in the case of a double bond, the molecule can exhibit different spatial arrangements, leading to the existence of geometric isomers.
What is the historical significance of the discovery of DNA's structure and how did it impact our understanding of genetics?
-The discovery of DNA's double helix structure was a monumental achievement in biology and genetics. It provided the physical basis for how genetic information is stored and replicated, which has profound implications for understanding heredity, gene expression, and the molecular basis of life. This understanding has been fundamental to the development of modern molecular biology and genetic engineering.
Outlines
🌟 Understanding 3D Molecular Structures
This paragraph introduces the concept of 3D molecular structures and their importance in organic chemistry. Deboki Chakravarti explains that organic molecules are not flat structures but can be plotted in a 3D space, which is crucial for understanding their interactions and functions. The paragraph discusses the historical development of theories such as Lewis structures, VSEPR, and hybridization, which have helped refine our understanding of molecular shapes. It emphasizes the significance of these shapes in predicting how molecules will interact, like pieces of a puzzle or an incompatible combination. The role of atomic orbitals and hybridization in explaining 3D geometries is also highlighted, using methane as an example to illustrate sigma bonds and the tetrahedral shape resulting from sp3 hybridization.
🔬 Valence Bond Theory and Molecular Geometry
The second paragraph delves into valence bond theory and its role in explaining the 3D shapes of molecules with double and triple bonds, specifically using ethene and ethyne as examples. It describes how carbon atoms in ethene hybridize their orbitals to form sp2 hybrid orbitals, leading to a trigonal planar geometry, and how the leftover p orbitals form a pi bond. For ethyne, sp hybridization results in a linear geometry due to the triple bond consisting of one sigma and two pi bonds. The paragraph also touches on the hybridization of other elements like oxygen in water molecules and carbonyl groups, and the historical significance of understanding these structures in deciphering the structure of DNA.
🔍 Isomers and Their Impact on Molecular Structure
The final paragraph discusses the concept of isomers, which are molecules with the same molecular formula but different arrangements of atoms. It differentiates between constitutional isomers, which have different connections between atoms, and geometric isomers, which have the same connections but different spatial arrangements. The paragraph uses octane and iso-octane as examples of constitutional isomers and explains how double bonds restrict free rotation, leading to geometric isomers. It concludes by emphasizing the importance of electron orbitals and atomic bonds in determining molecular shape and function, which are fundamental to all chemical reactions and life itself. The episode ends with a preview of future topics on understanding isomers and atom connections.
Mindmap
Keywords
💡Organic Chemistry
💡3D Molecular Structures
💡VSEPR Theory
💡Hybridization
💡Sigma and Pi Bonds
💡Isomers
💡Valence Bond Theory
💡DNA Structure
💡Ethene and Ethyne
💡Hybrid Orbitals
💡Constitutional Isomers
Highlights
Organic molecules have 3D shapes that are crucial for understanding their interactions and functions.
VSEPR theory explains the 3D shape of molecules based on electron pairs around a central atom.
There are five generally accepted VSEPR electron-pair geometries: linear, trigonal planar, tetrahedral, and two others.
Molecular shapes in organic chemistry often involve linear, trigonal planar, and tetrahedral geometries.
Water has a bent molecular shape due to its central oxygen atom's two lone pairs and two bonded hydrogens.
Orbital hybridization is a key concept that combines s and p orbitals to form hybrid orbitals, explaining 3D molecular geometries.
Methane's tetrahedral shape is a result of sp3 hybridization of carbon's valence electrons.
Sigma bonds are formed by the direct overlap of orbitals, as explained by valence bond theory.
Ethene's trigonal planar geometry is due to sp2 hybridization and the presence of a pi bond in addition to sigma bonds.
Ethyne's linear geometry is a result of sp hybridization and two pi bonds in addition to a sigma bond.
Other elements besides carbon, like oxygen, also exhibit hybridization and different electron-pair geometries.
The structure of DNA was elucidated with the help of valence bond theory and the concept of hybridization.
Isomers are molecules with the same molecular formula but different arrangements of atoms.
Constitutional isomers differ in the connections between atoms, while geometric isomers differ in the spatial arrangement of bonds.
Ethane can rotate around single bonds (free rotation), but double bonds lead to restricted rotation and the possibility of geometric isomers.
The prefixes cis and trans, or E and Z, are used to describe the spatial arrangement in geometric isomers.
Understanding molecular shapes and isomers is fundamental to all chemical reactions and biological processes.
Transcripts
Browse More Related Video

1.3 Valence Bond Theory and Hybridization | Organic Chemistry
Hybrid Orbitals explained - Valence Bond Theory | Orbital Hybridization sp3 sp2 sp

What Is Organic Chemistry?: Crash Course Organic Chemistry #1

General Chemistry Review for Organic Chemistry
1.4 Molecular Orbital Theory | Organic Chemistry

More Stereochemical Relationships: Crash Course Organic Chemistry #9
5.0 / 5 (0 votes)
Thanks for rating: