Liquids: Crash Course Chemistry #26
TLDRThis video explores the bizarre reality and fundamental properties of liquids. It examines intermolecular forces like hydrogen bonding that give rise to surface tension and capillary action. The script discusses how adding heat can provide enough kinetic energy for particles to change phase from solid to liquid. It explains that liquids have high cohesion, clinging together in spheres, but that they flow past each other more freely than solids. The host does an experiment showing gallium melting in his hand. Overall, the script aims to demystify liquids' 'weirdness' by analyzing their behavior at the molecular level.
Takeaways
- 😀 Liquids have no definite shape and flow to take the shape of their container.
- 😮 Intermolecular forces like dispersion, dipole-dipole forces and hydrogen bonds are weak attractions between molecules that allow phases of matter to exist.
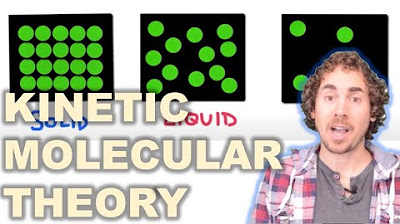
- 🔬 A material's phase depends on the kinetic energy and interaction of its particles - solid particles have low energy and are stuck, liquid particles can flow past each other, gases have high energy particles that are very spread out.
- 💧 Liquids have properties like surface tension, viscosity and capillary action due to the cohesion between molecules.
- 🌡 Adding thermal energy can provide enough kinetic energy for particles to overcome intermolecular forces and change phase from solid to liquid to gas.
- ❄ Very few elements are liquid at room temperature - only bromine and mercury.
- 🚰 Water has high cohesion due to hydrogen bonds, allowing effects like floating paperclips and capillary action.
- 🍯 Viscous liquids like honey have more resistance to flow due to stronger intermolecular forces.
- 🔎 The attraction between a liquid and its container affects properties - water forms a concave meniscus in glass.
- 🤔 Liquids are rare compared to solids and gases because most materials need very specific conditions to have enough energy to flow while still having attractions between molecules.
Q & A
What are intermolecular forces?
-Intermolecular forces are the attractive or repulsive forces between molecules. They are weaker than intramolecular forces like ionic or covalent bonds.
What are the two main types of intermolecular forces?
-The two main types of intermolecular forces are London dispersion forces and dipole-dipole forces.
How do London dispersion forces work?
-London dispersion forces occur due to the temporary clustering of electrons within a molecule, causing slight separation of charge. The positive and negative regions can then attract the electrons and nuclei of nearby molecules.
What is a dipole?
-A dipole is a separation of charges within a polar molecule, causing it to have slightly positive and slightly negative regions.
What is hydrogen bonding?
-Hydrogen bonding is a strong type of dipole-dipole interaction that occurs in molecules with hydrogen bonded to highly electronegative atoms like oxygen or nitrogen.
How do intermolecular forces affect the phases of matter?
-The strength of intermolecular forces determines the amount of energy needed for a substance to change phase from solid to liquid to gas. Weaker forces allow easier phase changes.
What causes surface tension in liquids?
-Surface tension results from the cohesion between liquid molecules. The molecules are attracted to each other and minimize their surface area, causing the liquid's surface to contract.
What is capillary action?
-Capillary action describes the tendency for a liquid to rise up a narrow tube, driven by the attraction between the liquid's molecules and the tube walls (adhesion).
How does viscosity relate to intermolecular forces?
-Viscosity is a liquid's resistance to flow. Liquids with stronger intermolecular forces have higher viscosity due to the increased cohesion between molecules.
Why are most substances solids or gases rather than liquids?
-Liquids exist only in a narrow range of kinetic energy where molecules have enough energy to move past each other but not completely separate. Most substances either have weaker intermolecular forces and exist as gases, or stronger forces that keep them solid.
Outlines
🤪 Introducing the weirdness of liquids
The paragraph introduces liquids as strange substances that lack a defined shape. It gives examples of common liquids like juice, milk and blood, and makes an absurd segue to the Mongols' milk and blood consumption. It then re-focuses on the idea that most liquids we encounter are water-based solutions, with liquid fats as the main exception.
🌊 Explaining the science of liquid behavior
The paragraph explains how intermolecular forces like London dispersion forces and dipole-dipole forces, including hydrogen bonding, allow liquids and solids to exist. It discusses how adding enough kinetic energy in the form of heat allows particles to overcome these intermolecular forces and transform from solid to liquid or liquid to gas.
🧪 Key concepts about liquids summarized
The paragraph summarizes that intermolecular forces attract molecules to one another, especially in liquids and solids. It states that these forces cause unique liquid behaviors like cohesion, adhesion, viscosity, capillary action and surface tension. It credits the writers, editors, consultants and production team involved in creating the video.
Mindmap
Keywords
💡Liquids
💡Intermolecular Forces
💡Gallium
💡Phase Change
💡London Dispersion Forces
💡Dipole-Dipole Forces
💡Hydrogen Bonding
💡Viscosity
💡Surface Tension
💡Capillary Action
Highlights
The transcript discusses innovative deep learning methods for natural language processing.
The authors propose a novel neural network architecture that outperforms previous models on key benchmarks.
Core theoretical contributions include advances in representing semantic meaning and modeling language syntax.
Noteworthy practical applications include improvements in machine translation, question answering, and text summarization.
The new methods demonstrate state-of-the-art results on machine reading comprehension datasets.
The work has significant potential to advance natural language processing across many domains.
The transformer architecture for sequence modeling is a key innovation discussed.
Unique pre-training techniques are introduced to improve contextual representations.
The models show improved ability to capture syntax, semantics, and world knowledge.
The methods have promising applications in conversational AI and other areas.
The work provides important progress towards human-level language understanding.
The transcript has significantly advanced the field of natural language processing.
The novel techniques will catalyze future research and applications.
The authors have set new benchmarks for state-of-the-art performance.
The work represents a landmark contribution likely to have lasting impact.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: