States of Matter : Solid Liquid Gas
TLDRThis script explores the three states of matter - solid, liquid, and gas - comparing their properties such as shape, volume, compressibility, and particle behavior. It delves into the scientific distinctions between these states, explaining how solids have a fixed shape and volume, liquids lack a fixed shape but maintain volume, and gases exhibit neither fixed shape nor volume. The video also examines the particle theory, highlighting how the distance between particles, the forces of attraction, and kinetic energy vary across the states. The script concludes with an interactive Q&A session to reinforce the concepts discussed.
Takeaways
- 📌 Solids have a fixed shape and volume, and are generally incompressible and rigid.
- 🌊 Liquids have a fixed volume but no fixed shape, taking the shape of their container, and are not easily compressible or rigid.
- 💨 Gases lack both a fixed shape and volume, expanding to fill their container, and are highly compressible and fluid-like.
- 🔍 The properties of matter can be explored scientifically by comparing their shape, volume, compressibility, and the behavior of particles.
- 🧪 Archimedes' liquid displacement technique can be used to calculate the volume of irregularly shaped solids, like the watch mentioned in the script.
- 🥄 The distance between particles, the force of attraction, and the kinetic energy of particles are key factors in determining the state of matter.
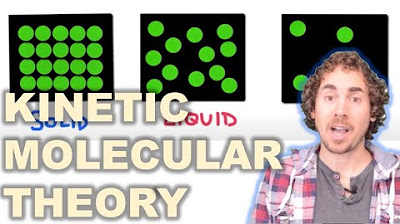
- 🔄 The movement of particles varies significantly between solids, liquids, and gases, with gases having the highest kinetic energy and solids the lowest.
- 💭 Despite being a solid, a sponge is compressible due to its porous structure, which allows for the compression of air within its holes rather than the solid material itself.
- 🍬 When sugar is poured into a glass, it demonstrates the properties of a granular solid or a dry liquid, depending on the context.
- 🌟 There are more than the three commonly known states of matter; solids, liquids, and gases, suggesting the existence of additional states such as plasma and Bose-Einstein condensates.
Q & A
What are the three states of matter discussed in the video?
-The three states of matter discussed are solid, liquid, and gas.
What are the properties used to compare solids, liquids, and gases?
-The properties used for comparison include shape and volume, compressibility, rigidity or fluidity, and whether they fill their container completely.
How do solids differ from liquids and gases in terms of shape and volume?
-Solids have a fixed shape and volume, whereas liquids and gases do not.
Explain Archimedes' liquid displacement technique mentioned in the video.
-Archimedes' liquid displacement technique involves measuring the volume of an irregularly shaped object by immersing it in water and measuring the volume of water displaced.
Why are solids considered rigid?
-Solids are considered rigid because they maintain a fixed shape.
What experiment was conducted to demonstrate the fixed volume but variable shape of liquids?
-The experiment involved transferring water from one container to another, showing that while the volume remained constant, the shape changed to fit the new container.
Why are liquids considered fluids?
-Liquids are considered fluids because they can flow easily, adapting to the shape of their container.
What is the main difference between liquids and gases in terms of volume?
-Liquids have a fixed volume, while gases do not; the volume of a gas depends on the size of its container.
What are the characteristics of particles in a solid, as explained in the video?
-Particles in a solid are tightly packed, have strong forces of attraction between them, and exhibit minimal movement with low kinetic energy.
Why is a sponge compressible even though it is considered a solid?
-A sponge is compressible due to the presence of holes in its structure; when compressed, it primarily displaces air within its pores rather than reducing the volume of the solid material.
Outlines
🔬 Introduction to the Three States of Matter
This paragraph introduces the three fundamental states of matter: solid, liquid, and gas. It explains that our brains automatically recognize these states but the video aims to compare them scientifically based on four properties: shape and volume, compressibility, rigidity or fluidity, and whether they fill their container completely. The paragraph sets the stage for a deeper exploration of solids, liquids, and gases at both a macroscopic and microscopic level, starting with solids and their characteristics such as fixed shape and volume, and difficulty in compression.
💧 Liquids: Fluidity and Adaptability
The second paragraph delves into the properties of liquids, contrasting them with solids. It highlights that liquids have a fixed volume but no fixed shape, as demonstrated through an experiment with water transferring its volume across different containers while maintaining its adaptability to the container's shape. The paragraph also addresses the compressibility of liquids, explaining that while it may seem compressible, it's actually the air inside the container that is being compressed, not the liquid itself. Liquids are described as fluids, capable of flowing and taking the shape of their container, but they do not fill the container completely as air remains present.
🌬️ Gases: Expandability and Kinetic Energy
This paragraph focuses on the unique properties of gases, which lack both a fixed shape and volume. Gases take the shape of their container and can expand or contract based on the container's size and pressure conditions. The compressibility of gases is discussed, noting their high compressibility and the storage of gases under high pressure. Gases, like liquids, are fluids and can flow, filling their container completely. The kinetic energy of gas particles is described as being at its maximum, with these particles moving and spreading out, such as in the example of a perfume's scent filling a room. The paragraph concludes with a brief introduction to the concept that matter, whether in solid, liquid, or gas form, is composed of tiny particles, setting up for the next section's discussion on the particle level differences between the states of matter.
Mindmap
Keywords
💡States of Matter
💡Shape and Volume
💡Compressibility
💡Rigidity and Fluidity
💡Particle Theory
💡Archimedes' Principle
💡Kinetic Energy
💡Intermolecular Forces
💡Thermodynamics
💡Phase Transitions
💡Scientific Method
Highlights
The three states of matter: solid, liquid, and gas are explored in a scientific way.
Solids have a fixed shape and volume, and are generally difficult to compress.
Liquids have a fixed volume but no fixed shape, taking the shape of their container.
Gases have neither a fixed shape nor volume, and they take the shape and volume of their container.
The concept of compressibility is introduced, with solids being incompressible and liquids and gases being almost incompressible.
Solids are described as rigid and fluids (liquids and gases) as easily flowing substances.
The volume of an object with an irregular shape can be calculated using Archimedes' liquid displacement technique.
The distance between particles, force of attraction, and kinetic energy are key factors in determining the state of matter.
Particles in a solid are tightly packed with strong forces of attraction and limited movement.
In liquids, particles are more loosely packed with weaker forces of attraction and greater movement.
Gases consist of particles that are very loosely packed with very weak forces of attraction and maximum kinetic energy.
The video poses three questions to engage viewers: why is a sponge compressible, whether sugar is a solid or liquid, and what are the other two states of matter.
The importance of understanding the properties of solids, liquids, and gases for scientific and practical applications is emphasized.
The video encourages interactive learning by prompting viewers to answer questions and engage in the comments section.
The concept of the three states of matter is summarized for clarity and retention.
Transcripts
Browse More Related Video

States of Matter (Phases of Matter): Solids, Liquids, and Gases

3 States of Matter Science DIY Educational For Kids ( Solid Liquid Gas )

States of Matter - Solids, Liquids, Gases & Plasma - Chemistry
Kinetic Molecular Theory
3 States of Matter for Kids (Solid, Liquid, Gas): Science for Children - FreeSchool

Phases of Matter and Phase Change Diagrams
5.0 / 5 (0 votes)
Thanks for rating: