10.1 Intermolecular Forces | High School Chemistry
TLDRThis chemistry lesson delves into intermolecular forces, crucial for understanding the properties of solids and liquids. It distinguishes between covalent bonds and weaker forces like London dispersion (Van der Waals), dipole-dipole interactions, and hydrogen bonding—the strongest of the three. The instructor emphasizes the significance of these forces in determining physical properties, such as boiling points, viscosity, and surface tension. The role of molecular size and polarity in intermolecular forces is highlighted, along with the impact of hydrogen bonding on water's unique behavior. The lesson also explores concepts like adhesion, cohesion, and capillary action, demonstrating their relevance in both chemistry and biology.
Takeaways
- 🔬 Intermolecular forces are the weak attractive forces between separate molecules, significantly weaker than covalent or ionic bonds.
- 🧲 The concept of 'inter' refers to 'between', highlighting that these forces occur between different molecules, not within them.
- ⚛️ Intermolecular forces are categorized into three main types: London dispersion forces (the weakest), dipole-dipole forces, and hydrogen bonding (the strongest).
- 🌐 London dispersion forces, also known as van der Waals forces, are present in all molecules due to the temporary dipoles created by the movement of electrons.
- 🔍 Dipole-dipole forces occur between polar molecules, where there is an attraction between the partially positive and partially negative ends of different molecules.
- 💧 Hydrogen bonding is a special type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like fluorine, oxygen, or nitrogen.
- 🌡 Properties such as boiling point, enthalpy of vaporization, viscosity, and surface tension are influenced by the strength of intermolecular forces, with stronger forces leading to higher values in these properties.
- 💦 Surface tension and capillary action are phenomena driven by intermolecular forces, particularly cohesion (attraction within the same substance) and adhesion (attraction to a different substance).
- 📉 Vapor pressure is negatively correlated with intermolecular forces; stronger intermolecular forces result in lower vapor pressure.
- 📚 The script also introduces ion-dipole forces, which are stronger than hydrogen bonding and occur in mixtures where an ionic compound is dissolved in a polar solvent.
- 📉 The boiling point of a liquid is defined as the temperature at which its vapor pressure equals the external pressure, which can be influenced by altitude.
Q & A
What are intermolecular forces?
-Intermolecular forces are the weak attractive forces between separate molecules. They are significantly weaker than covalent or ionic bonds and play a crucial role in determining the physical properties of substances, especially in solids and liquids.
Why are intermolecular forces weaker than covalent or ionic bonds?
-Intermolecular forces are weaker because they involve interactions between molecules rather than within a molecule. They result from temporary dipoles and are much weaker compared to the strong electron-sharing or electrostatic attractions in covalent or ionic bonds.
What are the three major categories of intermolecular forces discussed in the script?
-The three major categories of intermolecular forces are London dispersion forces (also known as van der Waals forces), dipole-dipole forces, and hydrogen bonding. Each category represents different strengths and types of interactions between molecules.
How do plus and minus charges relate to intermolecular forces?
-Intermolecular forces are largely influenced by the attraction between opposite charges, where pluses are attracted to minus. This principle is fundamental in chemistry and is the basis for the existence of dipole-dipole forces and hydrogen bonding.
What is the significance of hydrogen bonding in the context of intermolecular forces?
-Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when hydrogen is bonded to highly electronegative atoms like fluorine, oxygen, or nitrogen. It significantly influences the properties of molecules, especially water, leading to high boiling points, cohesion, and other unique properties.
Why is water considered a universal solvent in biology?
-Water is considered a universal solvent in biology primarily due to its hydrogen bonding. This strong intermolecular force allows water to dissolve a wide range of substances, making it essential for various biological processes and the structure of cells.
How does the strength of intermolecular forces affect the boiling point of a substance?
-The strength of intermolecular forces directly affects the boiling point of a substance. Stronger intermolecular forces require more energy to overcome, resulting in higher boiling points. Conversely, weaker forces lead to lower boiling points.
What is the relationship between intermolecular forces and vapor pressure?
-There is an inverse relationship between intermolecular forces and vapor pressure. Stronger intermolecular forces lead to lower vapor pressures because fewer molecules can escape the liquid phase to enter the vapor phase. Weaker forces result in higher vapor pressures.
How does the size of a molecule affect its London dispersion forces?
-London dispersion forces are affected by the size and surface area of a molecule. Larger molecules have greater surface areas, which allows for more extensive dispersion forces. Thus, larger molecules generally have stronger London dispersion forces compared to smaller ones.
What is the role of ion-dipole forces in solutions?
-Ion-dipole forces occur in solutions when an ionic compound is dissolved in a polar solvent, such as water. These forces are responsible for the attraction between the ions of the dissolved substance and the polar solvent molecules, and they can be stronger than hydrogen bonding. However, they are only present in mixtures, not in pure liquids.
How does the script explain the concept of vapor pressure in relation to temperature and boiling point?
-The script explains that vapor pressure increases with temperature due to the increased kinetic energy of molecules, allowing more molecules to escape into the vapor phase. The boiling point is defined as the temperature at which the vapor pressure of a liquid equals the external pressure, typically one atmosphere at sea level. At higher altitudes with lower external pressures, the boiling point decreases.
What are adhesion, cohesion, and capillary action, and how are they related to intermolecular forces?
-Adhesion is the attraction between molecules of a substance and another substance, such as water's attraction to glass. Cohesion is the attraction between molecules of the same substance, which causes water to bead up. Capillary action is the ability of a liquid to rise in narrow tubes due to adhesive and cohesive forces. All these phenomena are related to intermolecular forces, particularly hydrogen bonding and dipole-dipole interactions in the case of water.
Outlines
🔬 Intermolecular Forces in Chemistry
This paragraph introduces the concept of intermolecular forces, explaining that they are the forces between separate molecules. It contrasts these with the covalent bonds that hold molecules together, emphasizing that intermolecular forces are much weaker. The discussion also revisits the topic of ideal gases, clarifying that while they were previously described as having no attractive forces, there are indeed weak forces between molecules. The paragraph outlines three major categories of intermolecular forces: London dispersion forces (also known as van der Waals forces), dipole-dipole forces, and hydrogen bonding, with the latter being the strongest. The importance of opposite charges in chemical properties is highlighted, as is the attraction between positive and negative charges.
🔬 Dipole-Dipole Forces and Hydrogen Bonding
This paragraph delves deeper into dipole-dipole forces, explaining that these occur in polar molecules where there is a separation of charge, leading to partial positive and negative charges. The example of HCl is used to illustrate how the more electronegative chlorine atom becomes partially negatively charged, and the hydrogen partially positively charged. Hydrogen bonding is then introduced as a special case of dipole-dipole forces, occurring only with hydrogen atoms bonded to highly electronegative atoms like fluorine, oxygen, or nitrogen. The paragraph explains that hydrogen bonding is significantly stronger than other dipole-dipole forces and is crucial for understanding the unique properties of water, such as its high boiling point and expansion upon freezing.
🧊 The Significance of Hydrogen Bonding in Water
This paragraph focuses on the importance of hydrogen bonding in water, detailing how it affects water's properties such as boiling point and its expansion when frozen. The explanation includes how hydrogen bonds are mediated through the lone pair of electrons on the partially negative atom, and how water molecules can act as both hydrogen bond donors and acceptors. The discussion also touches on the crystalline structure of ice, where each water molecule is hydrogen-bonded to four others, leading to the expansion of water when it freezes.
🌡️ Intermolecular Forces and Their Impact on Physical Properties
This paragraph explores how intermolecular forces influence the physical properties of liquids, such as boiling point, enthalpy of vaporization, viscosity, and surface tension. It explains that stronger intermolecular forces lead to higher boiling points and enthalpies of vaporization, as more energy is required to overcome these forces. Viscosity is also linked to intermolecular forces, with higher forces resulting in a thicker fluid. Surface tension is discussed in the context of water bugs being able to walk on water due to the strong hydrogen bonding at the liquid's surface. The paragraph concludes by contrasting these properties with vapor pressure, which is negatively correlated with intermolecular forces.
🌡️ Vapor Pressure and Its Relationship with Intermolecular Forces
This paragraph discusses vapor pressure, explaining it as the measure of how many molecules have entered the gas phase above a liquid. It describes the equilibrium between the gas and liquid phases and how this is temperature-dependent. The paragraph clarifies that higher intermolecular forces lead to a lower vapor pressure, as fewer molecules can escape the liquid phase. The relationship between boiling point and vapor pressure is also explored, with the boiling point defined as the temperature at which a liquid's vapor pressure equals the external pressure.
🔍 Comparing Intermolecular Forces in Different Molecules
This paragraph provides an in-depth comparison of intermolecular forces in different molecules, focusing on hydrogen bonding, dipole-dipole forces, and London dispersion forces. It explains that molecules capable of hydrogen bonding will have the strongest intermolecular forces, followed by those with dipole-dipole forces, and then those with only London dispersion forces. The discussion includes examples of how these forces affect the boiling points and vapor pressures of molecules like CH3OH, CH3F, and CH4. The paragraph also introduces ion-dipole forces, which occur in mixtures when an ionic compound is dissolved in a polar solvent like water.
🌳 Capillary Action and Its Biological Significance
This paragraph concludes the lesson by introducing the concepts of adhesion, cohesion, and capillary action. It explains that cohesion refers to the attractive forces between molecules of the same substance, while adhesion is the attraction between molecules of different substances. Capillary action is described as the ability of a liquid to rise in narrow tubes due to the adhesive forces between the liquid and the tube material. Examples of capillary action in nature, such as water traveling up tree roots, are provided, highlighting the biological relevance of these concepts.
Mindmap
Keywords
💡Intermolecular Forces
💡Covalent Bonds
💡Polar Molecules
💡Dipole-Dipole Forces
💡Hydrogen Bonding
💡London Dispersion Forces
💡Boiling Point
💡Vapor Pressure
💡Viscosity
💡Surface Tension
💡Enthalpy of Vaporization
💡Ion-Dipole Forces
Highlights
Intermolecular forces are the focus of the lesson, explaining the forces between separate molecules in solids and liquids.
Intermolecular forces are categorized into three major types: London dispersion forces, dipole-dipole forces, and hydrogen bonding.
London dispersion forces, also known as van der Waals forces, are the weakest intermolecular forces present in all molecules.
Dipole-dipole forces occur between polar molecules with partial positive and negative charges attracting each other.
Hydrogen bonding is a strong intermolecular force involving hydrogen atoms bonded to highly electronegative atoms like F, O, or N.
Hydrogen bonding is crucial for the unique properties of water, including its high boiling point and expansion upon freezing.
The strength of intermolecular forces directly affects properties like boiling point, viscosity, and surface tension.
Vapor pressure is inversely related to intermolecular forces, with stronger forces leading to lower vapor pressure.
Ion-dipole forces are stronger than hydrogen bonding and occur in mixtures where ionic compounds are dissolved in polar solvents.
The relationship between vapor pressure and temperature is discussed, with higher temperatures leading to increased vapor pressure.
Boiling point is defined as the temperature at which a liquid's vapor pressure equals the external pressure.
At high altitudes, the reduced external pressure results in a lower boiling point for liquids.
Adhesion, cohesion, and capillary action are terms related to intermolecular forces, with applications in both chemistry and biology.
Cohesion refers to the attractive forces between molecules of the same substance, while adhesion is the attraction between different substances.
Capillary action is the movement of a liquid within a narrow space due to adhesive forces, with examples including the spreading of water in paper towels and water transport in trees.
The lesson concludes with a discussion on the practical applications of intermolecular forces in everyday phenomena and the importance of understanding these concepts.
Transcripts
Browse More Related Video

11.1 Intermolecular Forces | General Chemistry

1.6 Intermolecular Forces | Organic Chemistry

Van der Waals forces | States of matter and intermolecular forces | Chemistry | Khan Academy
AP Chemistry Unit 3 Review: Intermolecular Forces and Properties
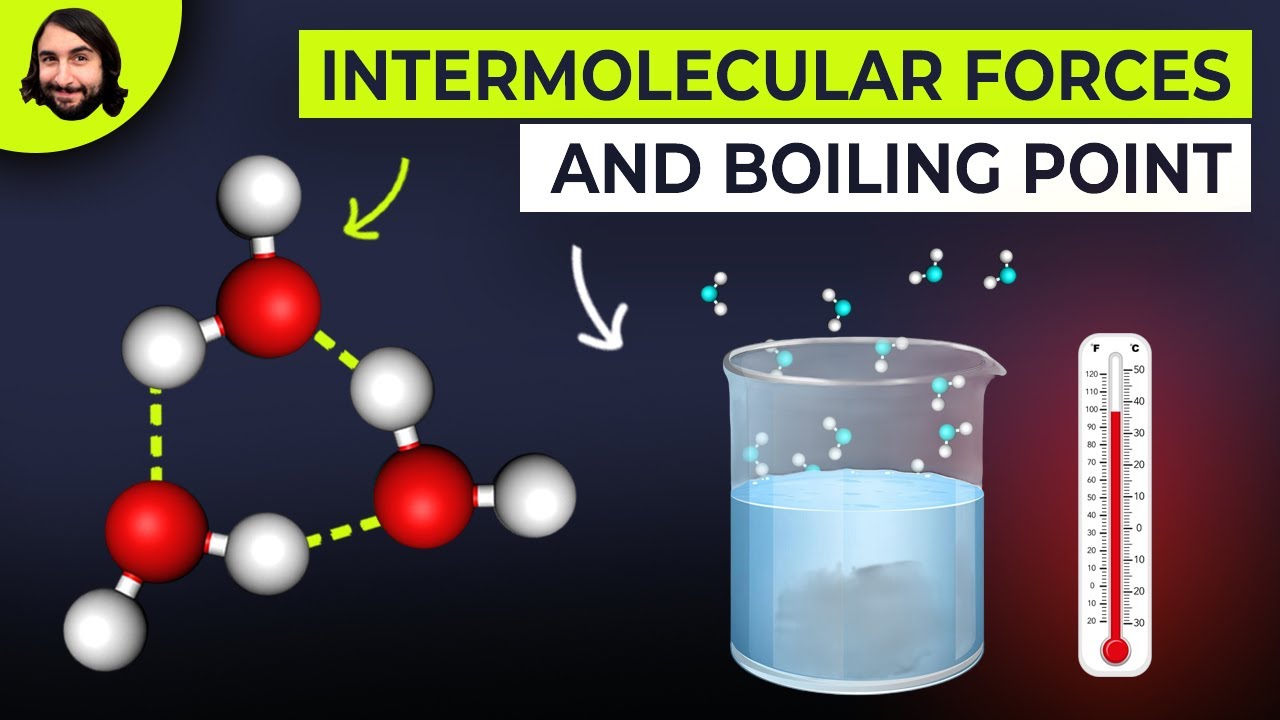
Intermolecular Forces and Boiling Points

AP Chem - Unit 3 Review - Intermolecular Forces & Properties
5.0 / 5 (0 votes)
Thanks for rating: