Acids and Bases for Kids | Learn the difference between an acid and a base
TLDRThis educational video introduces acids and bases to kids, using everyday examples like lemon juice and almond milk to illustrate their properties. It explains that acids are sour and have lots of hydrogen ions, while bases are bitter and have hydroxide ions. The video also covers the pH scale, which measures the acidity or alkalinity of a solution, and how acids and bases can neutralize each other. It highlights the importance of acids and bases in our daily lives, from digestion to household products.
Takeaways
- 🍋 Sour and bitter tastes are indicative of the presence of acids and bases in foods and drinks.
- 🧪 Lemon juice is an example of an acid, while almond milk is an example of a base.
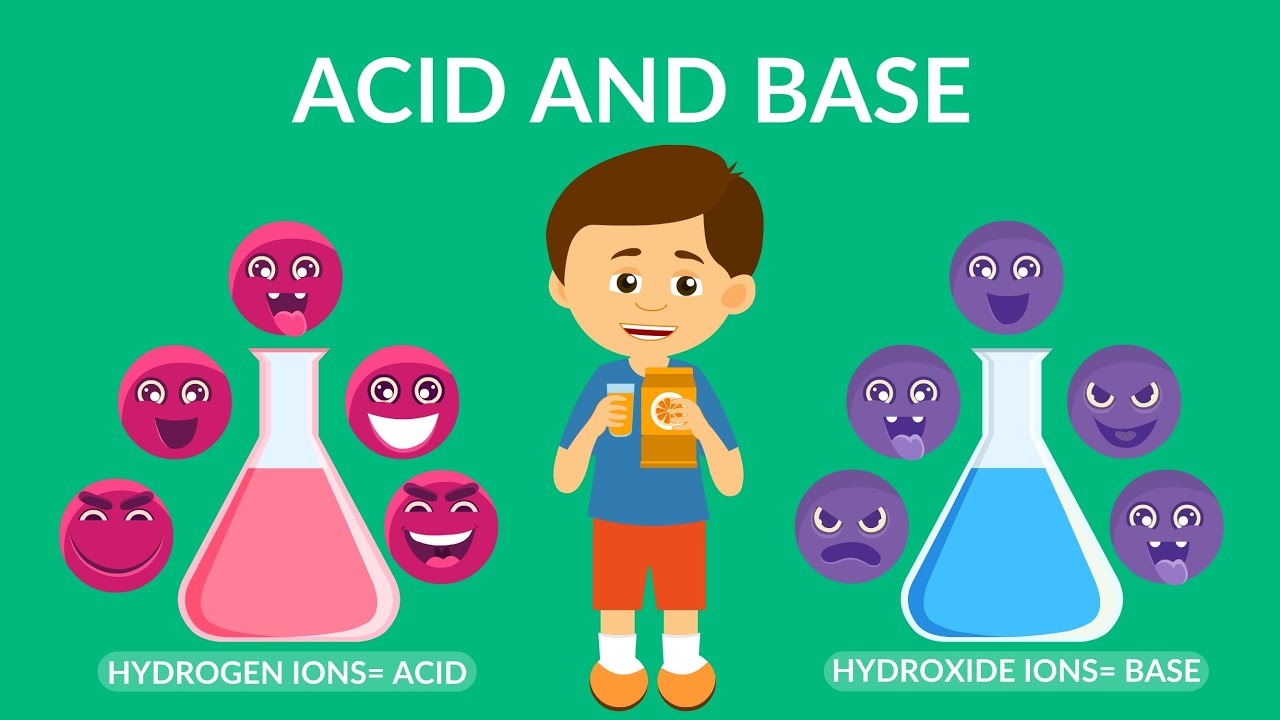
- 🌟 Acids are characterized by their high concentration of hydrogen ions, which makes them taste sour.
- 🥜 Bases, on the other hand, taste bitter and often have a soapy texture when touched.
- 📈 The pH scale is a tool used by scientists to measure the acidity or alkalinity of a solution based on the concentration of hydrogen ions.
- 🌊 Neutral pH is indicated by a score of 7, where the number of hydrogen ions and hydroxide ions are equal.
- 🛡️ Acids and bases can neutralize each other, providing a balance that can be beneficial in various applications such as digestion and dental care.
- 🍂 Natural indicators like litmus can be used to test the pH level of a substance, changing color in response to acids and bases.
- 🌿 Many everyday household products and foods are either acidic or basic, including vinegar, carbonated water, and lemon juice.
- 💡 Understanding acids and bases can enhance our knowledge of the world around us and their applications in our daily lives.
Q & A
What are the sour and bitter tastes caused by in foods and drinks?
-The sour and bitter tastes are caused by chemicals called acids and bases.
What two liquids were compared in the script to demonstrate the properties of acids and bases?
-Lemon juice and almond milk were compared to demonstrate the properties of acids and bases.
How does lemon juice taste and what does this indicate about its chemical composition?
-Lemon juice tastes sour, which indicates that it is an acid.
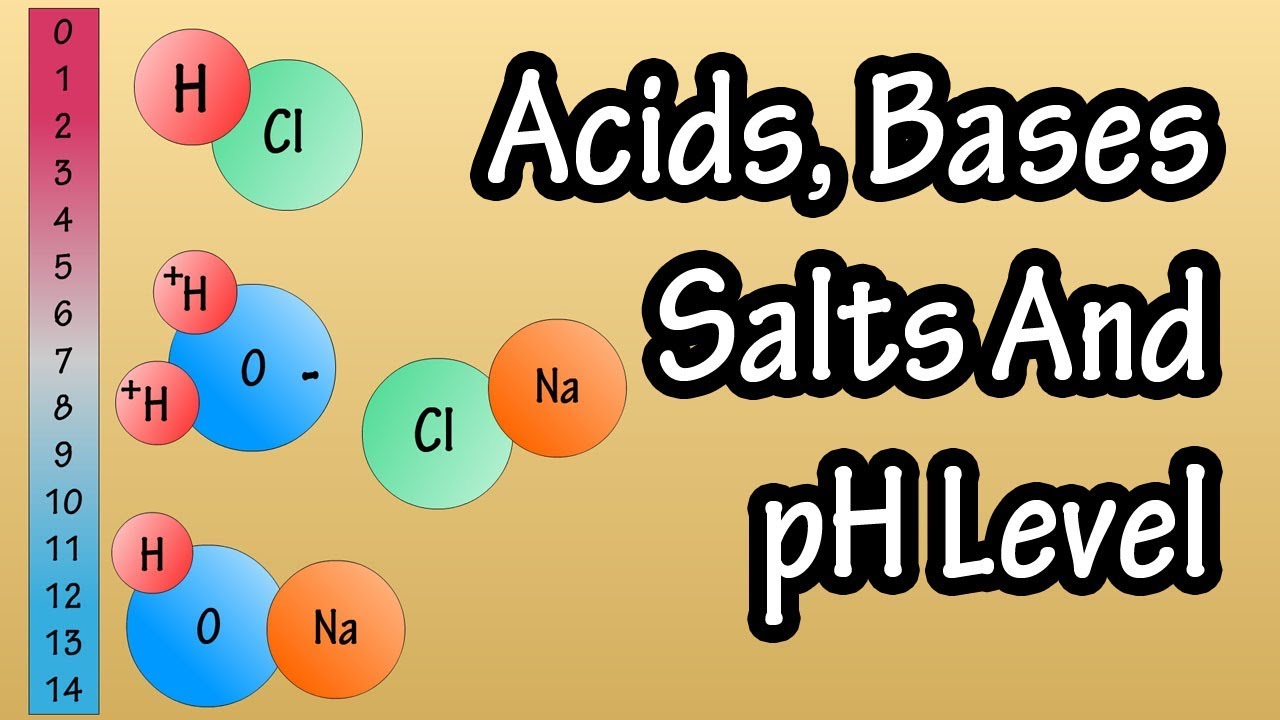
What is the significance of hydrogen ions in determining whether a substance is an acid or a base?
-Acids have a lot of hydrogen ions, while bases have more hydroxide ions. The presence and quantity of these ions determine the acidic or basic nature of a substance.
What is the role of hydrochloric acid in our bodies?
-Hydrochloric acid in our stomachs helps digest food and can also kill disease-causing germs.
What is the pH scale and how is it used to measure the level of acidity or alkalinity of a solution?
-The pH scale is a measurement system that ranges from 1 to 14 and is used to determine the level of acidity or alkalinity of a solution based on the number of hydrogen ions present.
What happens when acids and bases are combined?
-Acids and bases can neutralize or diffuse each other, resulting in a more balanced solution.
How can you tell if something is an acid or a base using an indicator?
-Indicators, such as litmus, can be used to determine if a substance is an acid or a base. Litmus turns red in acidic solutions and blue in basic solutions.
What are some common household items that are considered acids?
-Common household items that are acids include vinegar, carbonated water, lemon juice, and the acid in car batteries.
What is the role of a base produced by the pancreas in the body?
-The base produced by the pancreas helps with digestion and blood sugar regulation in the body.
What are some everyday examples of bases?
-Everyday examples of bases include baking soda, sugar, soaps, laundry detergents, and plant fertilizers.
Outlines
🍋 Introduction to Acids and Bases
This paragraph introduces the concepts of acids and bases, explaining their fundamental role in the taste of various foods and drinks. It highlights the sour taste of lemon juice as an example of an acid and the bitter taste of almond milk as an example of a base. The paragraph also sets the stage for a comparison between these two substances, discussing their appearance and taste. It further delves into the scientific explanation of acids, their prevalence in everyday life, and their high hydrogen ion content. The importance of acids in natural and human-made environments is emphasized, with examples ranging from stomach acid to household products.
📈 Understanding the pH Scale
This paragraph delves deeper into the scientific measurement of acids and bases through the pH scale. It explains the scale's range from 0 to 14, with acids falling below 7 and bases above 7, and a pH of 7 indicating a neutral solution. The paragraph highlights the dangers of strong acids and bases due to their reactivity and potential harm. It also discusses the concept of neutralization, where acids and bases can balance each other out, with practical examples such as milk of magnesia for an upset stomach and toothpaste to prevent tooth decay. The role of indicators, particularly litmus, in determining the pH level of a solution is introduced. The paragraph concludes with a review of the key points and encourages viewers to explore the presence of acids and bases in their surroundings, directing them to additional resources for learning.
Mindmap
Keywords
💡Acids and Bases
💡Taste
💡Hydrogen Ions
💡pH Scale
💡Neutralization
💡Indicators
💡Digestion
💡Soapy Texture
💡Hydroxide Ions
💡Litmus
💡Reactivity
Highlights
Acids and bases are chemicals that can affect the taste of food and drinks, such as sour lemon juice and bitter almond milk.
Lemon juice is a clear, yellow liquid that is sour to taste and is an example of an acid.
Almond milk is a cloudy, off-white liquid that is bitter to taste and is an example of a base.
Most liquids are either an acid or a base, with common examples including vinegar, carbonated water, and lemon juice.
Acids have a high concentration of hydrogen ions, which contributes to their sour taste and reactivity.
Bases have a higher concentration of hydroxide ions and often have a bitter taste and soapy texture.
The pH scale is used to measure the acidity or alkalinity of a solution, ranging from 1 to 14.
A score of 7 on the pH scale indicates a neutral solution, where the number of hydrogen ions equals the number of hydroxide ions.
Acids and bases can neutralize each other, such as milk or milk of magnesia neutralizing stomach acid.
Toothpaste is a base that helps neutralize the acid produced by decaying food in the mouth, preventing tooth decay.
Natural indicators like litmus can be used to test the pH level of a solution; it turns red in acidic solutions and blue in basic solutions.
Litmus is made from lichens, organisms that combine fungus and algae, and is typically found in the form of litmus paper.
Acids and bases are found in various everyday items, from food and drinks to cleaning supplies and batteries.
The concept of acids and bases is not only scientific but also has practical applications in health, digestion, and household maintenance.
Understanding acids and bases can lead to a better comprehension of chemical reactions and their role in our daily lives.
The Latin word 'acer' is the origin of the term 'acid,' reflecting the sour taste associated with many acids.
The pancreas produces a base that aids in digestion and blood sugar regulation, highlighting the importance of pH balance in the body.
Indicators like litmus are not only useful in scientific settings but also in everyday scenarios, such as testing the pH of natural substances.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: