pH and pOH: Crash Course Chemistry #30
TLDRThis video explains the concept of pH, which measures the acidity or alkalinity of a solution. It revolves around the dissociation of water molecules into hydronium and hydroxide ions, a reversible reaction described by the water dissociation constant Kw. The pH scale ranges from 0-14, with acids having lower pH and bases having higher pH. The pH and pOH, related to hydroxide ion concentration, always sum to 14. Logarithms allow convenient calculation of pH from hydrogen ion concentration. Strong vs weak acids and bases ionize to different degrees and so have different pH levels. Overall the video teaches the meaning, math, and applications behind the important chemical concept of pH.
Takeaways
- 😀 pH is defined as the negative log of the hydrogen ion concentration
- 🌿 Water can act as both an acid and a base by releasing and accepting protons
- 💧 The dissociation constant for water is called Kw
- 🔬 Strong acids and bases ionize more completely, weak acids and bases less so
- 📉 pH and pOH are inversely related through Kw
- 📊 pH below 7 is acidic, pH above 7 is basic, pH of 7 is neutral
- ⛑ Using logs lets us avoid very large or very small numbers
- 🌡 Indicators like litmus change color at different pH levels
- ⚖ The pH + pOH of any aqueous solution equals 14
- 🧪 Next video will show how to buffer pH against added acids/bases
Q & A
What does the 'p' in pH stand for?
-The 'p' in pH was likely derived from the French word 'puissance', meaning power, or from the Latin word 'pondus', also meaning power. However, the exact reasoning behind using a 'p' is unknown.
Why is water considered a neutral substance in terms of pH?
-Pure water is considered neutral, with a pH of 7, because the concentration of hydrogen (hydronium) ions and hydroxide ions are equal, at 1.0 x 10^-7 moles per liter.
What is the difference between a strong acid and a weak acid?
-A strong acid like hydrochloric acid ionizes completely in water, releasing many protons, thus having a very low pH. A weak acid like citric acid only partially ionizes, releasing fewer protons, and thus tends to have a higher pH in the 4-6 range.
How is pOH related to pH?
-The pOH is the negative log of the hydroxide ion concentration. The sum of pH and pOH always equals 14 for any aqueous solution.
Why does the pH scale use logarithms?
-Logarithms allow the pH scale to deal with very large or very small numbers by converting them into manageable values. Instead of tiny concentrations like 10^-7, we can use the logarithm, 7.
What causes water molecules to form hydronium and hydroxide ions?
-Random electrical field changes around water molecules cause them to temporarily break apart into H3O+ and OH- ions, before rapidly reforming into water again. This occurs constantly.
What is an equilibrium constant?
-An equilibrium constant is a ratio relating the product concentrations to reactant concentrations for a reversible reaction, all raised to the power of their coefficients.
What does Kw stand for and what is its value?
-Kw stands for the water dissociation constant. Its value is 1.0 x 10^-14, and it represents the ion product of water's dissociation into hydronium and hydroxide ions.
What causes some pH indicators like litmus to change color?
-pH indicators contain chemicals that change color depending on the pH. Acids cause one color change, bases cause a different color change.
How can you keep the pH stable when adding acids or bases?
-You can use a buffer solution, which resists pH change on addition of acid or base by absorbing or releasing protons.
Outlines
😊 What is pH and why is it important?
This paragraph explains what pH is, why it is written with a lowercase 'p' and uppercase 'H', and introduces its significance in relation to acids, bases and chemical reactions. It touches on pH balance and equilibrium states. The pH scale measures the acidity or alkalinity of a solution on a scale from 0 to 14, with 7 being neutral.
😀 How water molecules dissociate into ions
This paragraph discusses the dissociation of water molecules into hydronium and hydroxide ions, which allows water to act as both an acid and base. It introduces the water dissociation constant (Kw) and equilibrium concentrations of the ions, explaining how this leads to the pH scale with neutral pH being 7.
🤓 Mathematical connections between pH and pOH
This closing paragraph notes some mathematical connections between pH and pOH, specifically that their sum is always 14. It also recap key learning from the video including equilibrium reactions in water, strong vs. weak acids/bases, using logarithms to calculate pH, and the meanings of pH and pOH.
Mindmap
Keywords
💡pH
💡acids
💡bases
💡litmus paper
💡logarithms
💡water dissociation
💡hydronium ion
💡hydroxide ion
💡equilibrium
💡neutralization
Highlights
pH represents the power of hydrogen in a solution.
The 'H' in pH stands for hydrogen, as hydrogen ions are key to acid/base behavior.
Water can act as both an acid and a base by releasing and accepting protons.
Kw is the water dissociation constant, defining the equilibrium concentrations of H+ and OH- ions.
The pH scale ranges from 0-14, with acids below 7 and bases above 7.
Strong acids fully ionize to release lots of H+, having very low pH.
Weak acids partially ionize, releasing fewer H+ ions and having higher pH.
Strong bases consume many H+ ions, having high pH.
Weak bases consume fewer H+ ions, with pH 8-11.
Neutral pH is between 6-8.
pOH is the negative log of the OH- concentration.
The sum of pH and pOH always equals 14.
Next week: how to hold pH steady when adding acids/bases.
Learned about water's ionization, acids, bases, pH/pOH calculations.
Logarithms let us calculate pH from H+ concentration without huge numbers.
Transcripts
Browse More Related Video
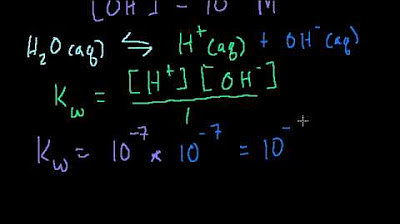
Introduction to pH, pOH, and pKw
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained

pH, pOH of strong acids and bases | Chemistry | Khan Academy
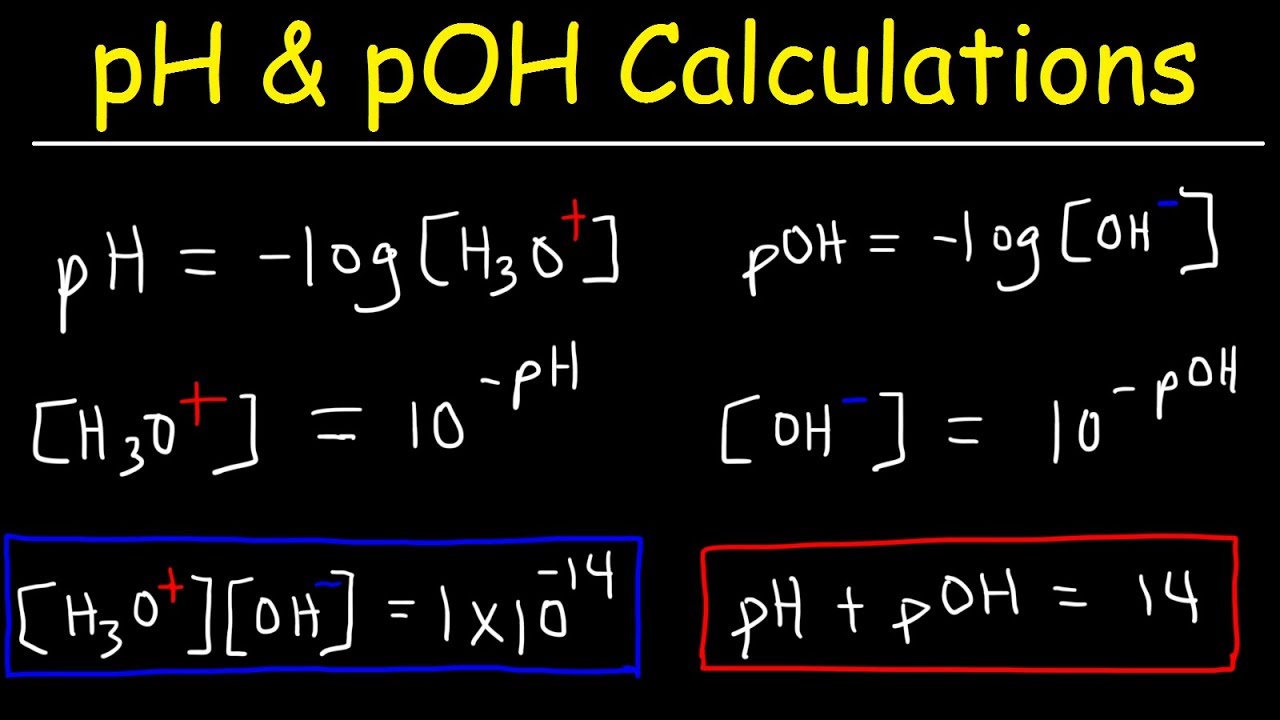
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems

What Are Acids & Bases? | Chemistry Basics

Acids, Bases, and pH
5.0 / 5 (0 votes)
Thanks for rating: