pKa and pKb relationship | Acids and bases | Chemistry | Khan Academy
TLDRThe video script delves into the chemistry of weak acids and bases, explaining their dissociation in aqueous solutions and the equilibrium they establish. It introduces the concept of conjugate acids and bases, and their respective equilibrium constants, Ka and Kb. The script further explores the relationship between Ka and Kb, culminating in the revelation that their sum equals 14 at room temperature in aqueous solutions, a principle pivotal for understanding acid-base chemistry.
Takeaways
- 🔍 The script discusses the concept of weak acids and their dissociation in aqueous solutions, emphasizing the equilibrium state between the acid and its conjugate base.
- 🌡 It explains that the equilibrium constant for a weak acid, denoted as Ka, is the product of the concentrations of hydrogen ions and the conjugate base divided by the concentration of the weak acid.
- 📉 The script also introduces the concept of a weak base and its equilibrium constant, denoted as Kb, which is the product of the concentrations of the weak base and hydroxide ions divided by the concentration of the conjugate acid.
- 🔄 The relationship between the acid and its conjugate base is highlighted, showing that the same reaction can be written in two different ways, reflecting both acidic and basic perspectives.
- ⚖️ The script demonstrates how to derive a relationship between Ka and Kb, showing that their product equals the product of the hydrogen ion concentration and the hydroxide ion concentration in an aqueous solution.
- 🌟 The importance of the water autoionization constant, Kw, is emphasized, which is the equilibrium constant for the self-ionization of water, and is always 10^-14 at 25 degrees Celsius.
- 🤔 The script uses the relationship between Ka and Kb to show that the sum of the negative logarithms of these constants, or pKa and pKb, equals 14, a useful tool for calculating unknown equilibrium constants.
- 🧪 An example is given to illustrate how knowing the pKa of a weak acid can be used to calculate the pKb of its conjugate base, and vice versa.
- 📚 The script provides a clear understanding that the concepts of Ka, Kb, and their relationship to Kw are fundamental to the study of acid-base chemistry.
- 📉 The explanation of how the equilibrium constants for weak acids and bases are derived from the concentrations of ions in solution is a key takeaway, showing the dynamic nature of these equilibria.
- 🔬 The practical application of these concepts is underscored, with the script showing how to use the derived relationships to understand and predict the behavior of weak acids and bases in solution.
Q & A
What is the general form of a weak acid in an aqueous solution?
-The general form of a weak acid in an aqueous solution is HA, where H+ represents the hydrogen ion and A- represents the rest of the acid molecule that retains the electrons.
What is the equilibrium reaction for a weak acid and how is it represented?
-The equilibrium reaction for a weak acid is represented as HA ⇌ H+ + A-, indicating that the weak acid can dissociate into a hydrogen ion and its conjugate base in an aqueous solution.
What is the difference between a weak acid and a strong acid in terms of their reactions in solution?
-A weak acid establishes an equilibrium in solution and does not completely dissociate, whereas a strong acid fully dissociates in solution, releasing all its hydrogen ions and not establishing an equilibrium.
What is the conjugate base of a weak acid, and how does it relate to the acid?
-The conjugate base of a weak acid is the species formed after the weak acid donates a proton (H+). It is represented as A- and is in equilibrium with the weak acid in solution.
What is the significance of the equilibrium constant Ka in the context of weak acids?
-The equilibrium constant Ka represents the tendency of a weak acid to donate a proton in an aqueous solution. It is calculated as the product of the concentrations of H+ and A- divided by the concentration of HA.
How is the equilibrium constant for the basic reaction of a conjugate base represented and what is it called?
-The equilibrium constant for the basic reaction of a conjugate base is represented as Kb and is calculated as the product of the concentrations of HA and OH- divided by the concentration of A-.
What is the relationship between the equilibrium constants Ka and Kb for a weak acid and its conjugate base?
-The relationship between Ka and Kb is given by the equation Ka × Kb = [H+] × [OH-], which equals 10^-14 in an aqueous solution at 25 degrees Celsius.
What is the significance of the constant 10^-14 in the context of aqueous solutions at 25 degrees Celsius?
-The constant 10^-14 is the product of the concentrations of hydrogen ions [H+] and hydroxide ions [OH-] in an aqueous solution at 25 degrees Celsius, representing the ionic product of water (Kw).
What is the pKa and how is it related to the Ka of a weak acid?
-The pKa is the negative logarithm of the acid dissociation constant (Ka). It is used to express the acidity of a weak acid on a logarithmic scale.
What is the pKb and how can it be determined if the pKa of a weak acid is known?
-The pKb is the negative logarithm of the base dissociation constant (Kb) for the conjugate base of a weak acid. It can be determined using the relationship pKa + pKb = 14.
How can the relationship between pKa and pKb be used to find the Kb of a conjugate base if the pKa of its corresponding weak acid is known?
-If the pKa of a weak acid is known, the pKb of its conjugate base can be found by subtracting the pKa from 14, since pKa + pKb = 14.
Outlines
🔬 Weak Acid Equilibrium and Conjugate Base Reactions
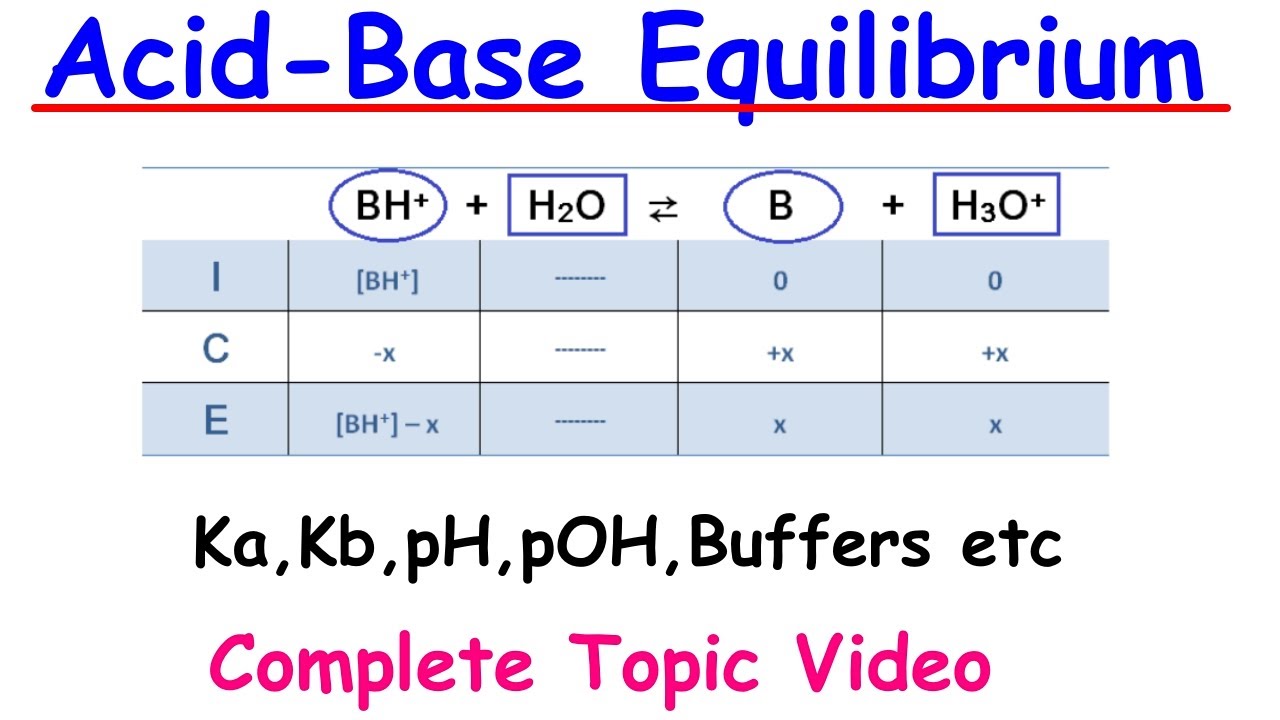
The script begins with an exploration of weak acids in an aqueous solution, explaining the concept of equilibrium between the acid (HA) and its dissociated form (H+ and A-). It uses the example of NH3 and HF to illustrate how different molecules can act as weak acids. The video then transitions into discussing the conjugate base (A-) and its ability to accept a proton from water to form a neutral molecule and hydroxide ions (OH-). The equilibrium constants for both the acidic (Ka) and basic (Kb) reactions are introduced, emphasizing that these constants are applicable only to weak acids and bases. The script also explains that strong acids and bases do not have equilibrium constants because they do not establish equilibrium but rather react completely.
📚 Establishing the Relationship Between Ka and Kb
This paragraph delves into the mathematical relationship between the equilibrium constants Ka and Kb. It starts by setting up expressions for the concentrations of the conjugate base (A-) in terms of Ka and Kb. The script then demonstrates how these expressions can be equated due to the identical nature of the reactions being described. By manipulating these equations, the video derives a formula that relates Ka and Kb through the concentration of hydrogen ions (H+). The relationship is further simplified by introducing the concept of the ionic product of water (Kw), which is a constant at 25 degrees Celsius in an aqueous solution, leading to the conclusion that the product of Ka and Kb equals Kw.
📉 pKa and pKb: The Logarithmic Relationship and Its Utility
The final paragraph discusses the utility of the relationship between pKa and pKb, which are the negative logarithms of Ka and Kb, respectively. It explains how the sum of pKa and pKb equals 14 at room temperature in an aqueous solution, a fact that is derived from the product of hydrogen and hydroxide ion concentrations being constant at 10^-14. The script uses the example of ammonium (NH4+) to show how knowing the pKa of a weak acid allows one to calculate the pKb of its conjugate base, and vice versa. This relationship is highlighted as a valuable tool in understanding and predicting the behavior of weak acids and bases in solution.
Mindmap
Keywords
💡Weak Acid
💡Equilibrium
💡Conjugate Base
💡Aqueous Solution
💡Equilibrium Constant (Ka, Kb)
💡Autoionization
💡Hydrogen Ion (H+)
💡Hydroxide Ion (OH-)
💡pKa and pKb
💡Autoionization Constant (Kw)
Highlights
Introduction to weak acids and their equilibrium with hydrogen and the rest of the molecule.
Explanation of the standard convention for representing the rest of the acid molecule as A-.
Illustration of the aqueous solution and the formation of H+ and A- ions.
Examples of different weak acids, including NH3 and HF, and their dissociation in water.
Concept of conjugate base formation from weak acids.
Equilibrium reactions for both the acidic and basic forms of the weak acid.
Introduction of the equilibrium constants Ka and Kb for weak acids and bases.
Differentiation between equilibrium reactions of weak and strong acids or bases.
Demonstration of finding the relationship between Ka and Kb using the same A- ion.
Derivation of the relationship Ka/[H+] = [OH-]/Kb.
Explanation of the autoionization of water and the equilibrium constant Kw.
The significance of the product of [H+] and [OH-] always being 10^-14 in aqueous solutions.
Derivation of the equation relating Ka and Kb: Ka * Kb = [H+] * [OH-].
Conversion of the relationship to the logarithmic form: pKa + pKb = 14.
Practical application of the pKa and pKb relationship to find unknown equilibrium constants.
Example calculation using the pKa of NH4+ to determine the pKb of NH3.
Emphasis on the constant value of pKa + pKb = 14 in aqueous solutions at room temperature.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: