16.5 pH Calculations for Weak Acids and Bases | General Chemistry
TLDRThis lesson delves into the calculations of pH for weak acids and bases, offering a comprehensive understanding of their dissociation in water. The instructor, Chad, explains the process of determining the H+ concentration for weak acids using the Ka value and provides a shortcut for calculations. A similar method is applied for weak bases, utilizing the Kb value. The lesson also addresses the relationship between Ka and Kb values for conjugate acid-base pairs, highlighting how the strength of an acid correlates with the weakness of its conjugate base and vice versa. Practical examples and step-by-step calculations are provided, making the content accessible for students.
Takeaways
- 📚 The lesson focuses on pH calculations for weak acids and bases, aiming to simplify the process of understanding their dissociation in solutions.
- 🔍 Weak acids partially dissociate in solution, resulting in a H+ concentration less than the initial acid concentration, leading to a pH greater than 1.
- 🧪 The Ka expression (equilibrium constant for weak acid dissociation) is crucial for calculating the pH of a weak acid solution.
- 📈 The Ka value reflects the strength of a weak acid, with higher Ka values indicating stronger acids and greater degrees of dissociation.
- 📊 A comparison of Ka values allows for the relative strength assessment of different weak acids, with the stronger acid having the higher Ka value.
- 🤔 The pH of a weak acid solution is determined through a more complex calculation than that of a strong acid, often involving an ICE table to find the equilibrium concentration of H+.
- 🎯 A shortcut for weak acid pH calculation involves using the Ka value and the initial acid concentration, taking the square root of their product to find the H+ concentration, then calculating the pH.
- 🧬 For weak bases, the process is analogous but involves the Kb expression (equilibrium constant for weak base dissociation) and results in the calculation of POH instead of pH.
- 🌟 The relationship between a weak acid and its conjugate base is such that the stronger the acid, the weaker the conjugate base, and vice versa.
- 🔢 Given the pH of a weak base solution, one can calculate the Kb value by understanding the dissociation equation and setting up the corresponding equilibrium expression.
- 🚀 Practice and familiarity with these concepts and formulas are essential for mastering pH calculations, which are integral to understanding acid-base chemistry.
Q & A
What is the main topic of this lesson?
-The main topic of this lesson is the pH calculations for weak acids and bases.
What is the difference between strong acids and weak acids in terms of dissociation?
-Strong acids dissociate completely, while weak acids only partially dissociate in solution.
What is the role of Ka in the context of weak acids?
-Ka is an equilibrium constant specifically for when a weak acid dissociates, and it helps determine the relative strength of the acid.
How can you calculate the pH of a weak acid solution using the Ka value?
-You can calculate the pH of a weak acid solution by using the formula: pH = -log[H+], where [H+] is determined from the Ka expression and the initial concentration of the acid.
What is the general formula for the dissociation of a weak base in water?
-The general formula for the dissociation of a weak base in water is: B + H2O ⇌ BH+ + OH-.
What is the relationship between Ka and Kb for a conjugate acid-base pair?
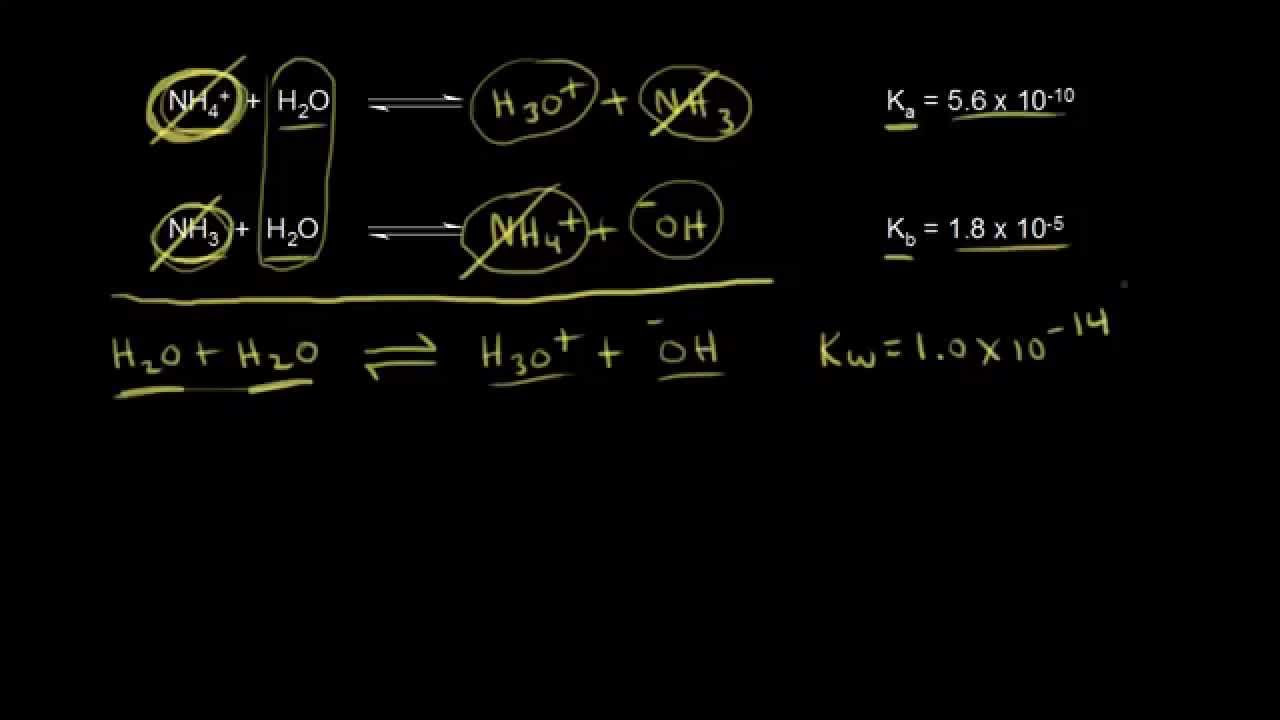
-For a conjugate acid-base pair, the product of the Ka of the acid and the Kb of the base equals the ion product of water (KW), which is 1.0 × 10^-14 at 25°C.
How can you find the Kb value of a weak base given its pH?
-You can find the Kb value of a weak base from its pH by first calculating the pOH (pOH = 14 - pH), and then finding the hydroxide ion concentration ([OH-]) from the pOH. Finally, use the Kb expression with [OH-] and the base concentration to solve for Kb.
What is the significance of the equilibrium constant (Ka or Kb) in predicting the strength of an acid or a base?
-The equilibrium constant (Ka for acids, Kb for bases) indicates the strength of an acid or a base. A higher Ka value means a stronger acid, while a higher Kb value indicates a stronger base. The constants are used to compare the relative strengths of different acids or bases.
How does the percent dissociation of a weak acid or base relate to its equilibrium constant?
-The percent dissociation of a weak acid or base is related to its equilibrium constant in that a larger Ka or Kb value indicates a greater degree of dissociation and thus a stronger acid or base. The percent dissociation can be calculated as the amount dissociated divided by the initial concentration, multiplied by 100.
What is the process for calculating the pH of a weak base solution?
-To calculate the pH of a weak base solution, you first find the hydroxide ion (OH-) concentration using the Kb expression and the initial concentration of the base. Then, you calculate the pOH by taking the negative logarithm of the OH- concentration. Finally, you determine the pH by subtracting the pOH from 14.
What is the role of water in the dissociation of a weak base?
-In the dissociation of a weak base, water acts as a proton donor. When the weak base (B) accepts a proton (H+) from water, it forms its conjugate acid (BH+) and leaves behind a hydroxide ion (OH-) in the solution.
How can you determine the type of solution you are dealing with for pH calculations?
-To determine the type of solution for pH calculations, you need to identify whether it is a strong acid, strong base, weak acid, weak base, buffer, or undergoing a titration. Each type of solution requires a specific method for calculating pH.
Outlines
📚 Introduction to pH Calculations for Weak Acids and Bases
The video begins with an introduction to the topic of pH calculations for weak acids and bases. The instructor, Chad, welcomes viewers to 'Chad's Prep' and outlines the services offered, including high school and college science prep, as well as MCAT, DAT, and OAT preparation. He introduces the lesson as part of a general chemistry playlist and encourages viewers to subscribe for updates. The lesson focuses on the differences between strong and weak acids, highlighting that weak acids only partially dissociate, leading to a pH greater than one. Chad explains the need for additional information, such as the Ka expression and value, to determine the exact pH of a weak acid solution.
🧪 Understanding the Equilibrium Constant (Ka) for Weak Acids
This paragraph delves deeper into the concept of the equilibrium constant (Ka) for weak acids. Chad explains that the strength of an acid is measured by its degree of dissociation, with a higher dissociation leading to a higher Ka value. He provides examples of three weak acids with their respective Ka values, allowing viewers to compare their relative strengths. The discussion transitions into how to calculate the pH of a weak acid solution using the Ka value and the initial concentration of the acid. Chad emphasizes the complexity of this process compared to strong acids but promises to introduce a shortcut method for most weak acids.
📊 Solving for pH of a Weak Acid Solution
Chad presents a step-by-step process for calculating the pH of a 0.1 molar solution of acetic acid as an example. He introduces the generic Ka expression and explains how to set up an ICE (Initial, Change, Equilibrium) table to determine the equilibrium concentration of H+ ions. The calculation involves simplifying the Ka expression by ignoring the minus X term due to the small amount of dissociation in weak acids. Chad demonstrates how to solve for X, which represents the equilibrium concentration of H+, and then how to use this value to calculate the pH. He also mentions that a shortcut exists for such calculations, which will be discussed later in the lesson.
🔄 Solving for Ka Given the pH of a Weak Acid
In this section, Chad addresses a different scenario where the pH of a weak acid solution is known, and the goal is to find the Ka value. Using the pH value, he explains how to determine the equilibrium concentration of H+ ions and subsequently calculate the Ka value. He reiterates the process of setting up the ICE table and solving the Ka expression, but this time including the minus X term for accuracy. Chad then calculates the Ka value and discusses the percentage dissociation of the weak acid, providing a method to determine how much of the acid has dissociated based on the initial concentration and the equilibrium concentration of H+.
🧪 Introduction to Weak Bases and their Equilibrium Constant (Kb)
Chad transitions from weak acids to weak bases, explaining the difference in their dissociation reactions and the involvement of water. He introduces the equilibrium constant for weak bases, known as Kb, and explains its relationship with the Ka of the conjugate acid. The discussion includes the Bronsted-Lowry definition of a weak base as a proton acceptor and the necessity of including water in the reaction equation. Chad sets up the generic reaction for a weak base dissociating in water and introduces the Kb expression, drawing parallels to the process followed for weak acids.
📊 Solving for pH of a Weak Base Solution
Chad demonstrates how to calculate the pH of a weak base solution, specifically a 0.1 molar solution of ammonia with a given Kb value. He outlines the process of setting up an ICE table for the weak base and solving for the hydroxide ion (OH-) concentration. The calculation involves a similar approach to that used for weak acids but with a focus on OH- instead of H+. Chad shows how to solve for X, representing the hydroxide concentration, and then how to convert this to POH and subsequently calculate the pH by subtracting from 14. He emphasizes the extra step required for weak bases compared to weak acids and reiterates the process for clarity.
🔄 Solving for Kb Given the pH of a Weak Base
Chad tackles a more challenging problem: finding the Kb value of a weak base when the pH of a 0.1 molar solution is known. He guides viewers through the process of converting the pH to POH, determining the hydroxide ion concentration, and then using this information to solve for Kb. The calculation requires substituting the known values into the Kb expression and accounting for the minus X term. Chad calculates the Kb value, highlighting the weak nature of the base in question. He reinforces the importance of understanding the differences in calculating pH for weak acids and bases and the relationship between their respective Ka and Kb values.
📚 Summary of pH Calculations for Different Types of Solutions
In the concluding paragraph, Chad summarizes the key points from the lesson, emphasizing the importance of understanding the different types of solutions and their corresponding pH calculation methods. He outlines the processes for calculating the pH of strong acids and bases, weak acids and bases, and hints at upcoming topics such as buffers and titrations. Chad encourages viewers to practice and master pH calculations, providing resources for further study and practice. He also invites viewers to engage with the content by using the thumbs up button and explores additional study materials through the provided links.
Mindmap
Keywords
💡Weak Acids
💡pH
💡Ka
💡Dissociation
💡Conjugate Base
💡ICE Table
💡Weak Bases
💡Kb
💡POH
💡Equilibrium Constant
💡Titration
Highlights
Chad introduces the topic of pH calculations for weak acids and bases, aiming to simplify the learning process.
The lesson is part of a general chemistry playlist, with new lessons released weekly throughout the school year.
Chad reviews the concept of strong acids, using 0.1 molar HCL as an example of complete dissociation leading to a quick pH calculation.
The difference between strong and weak acids is highlighted, with weak acids only partially dissociating and requiring more complex calculations for pH.
Chad explains the need for the Ka expression and Ka value to determine the pH of weak acids, which is an equilibrium constant for weak acid dissociation.
The relationship between the Ka value and the strength of an acid is discussed, with higher Ka values indicating stronger acids.
Chad provides a generic method for writing the dissociation reaction and Ka expression for any given weak acid.
The process of calculating the pH of a 0.1 molar solution of acetic acid is detailed, using an ICE Table and quadratic equation avoidance strategies.
A shortcut for calculating the pH of weak acids is introduced, which involves the square root of the product of the Ka value and the initial acid concentration.
Chad demonstrates how to find the Ka value of a weak acid if the pH of a solution is given, using a reverse calculation approach.
The concept of percent dissociation for weak acids is explained, with a calculation method provided based on the equilibrium concentration of H+.
The lesson transitions to weak bases, with a预告 of an analogous process and the introduction of the Kb expression for weak base dissociation.
The importance of including water in the reaction for weak bases is discussed, as it is essential for the base to accept a proton from water.
The relationship between Ka and Kb values for conjugate acid-base pairs is revealed, with the product always equaling the water ionization constant, KW.
Chad shows how to calculate the pH of a weak base solution using the Kb value, with an example calculation for a 0.1 molar solution of ammonia.
The process for finding the Kb value of a weak base, given the pH of a solution, is outlined, with an example problem provided.
Chad emphasizes the importance of recognizing the type of solution (strong acid, strong base, weak acid, weak base, buffer, or titration) for accurate pH calculations.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: