Ka and Kb
TLDRIn this insightful episode of 'Teach Me, The Science,' Sarin dives into the fundamental concepts of Ka and Kb, essential for understanding acid-base equilibrium. Starting with a primer on equilibrium constants, Sarin explains Ka and Kb as specialized versions of these constants, focusing on their roles in describing the dissociation levels of weak acids and bases. Viewers are guided through the derivation of Ka and Kb expressions, their practical applications, and when to use each. The tutorial also demystifies how to interconvert Ka and Kb values using the water dissociation constant (Kw). Sarin's clear explanations make complex chemistry concepts accessible, promising valuable knowledge for students grappling with acid-base chemistry.
Takeaways
- 📚 KA and KB are equilibrium constants related to acid-base equilibrium, specifically for weak acids and bases.
- 🔄 Equilibrium constants are the ratio of products to reactants at equilibrium.
- 🧪 The dissociation of a weak acid is represented by the KA expression, which involves the concentration of the acid (A-), hydronium ion (H3O+), and the undissociated acid (HA).
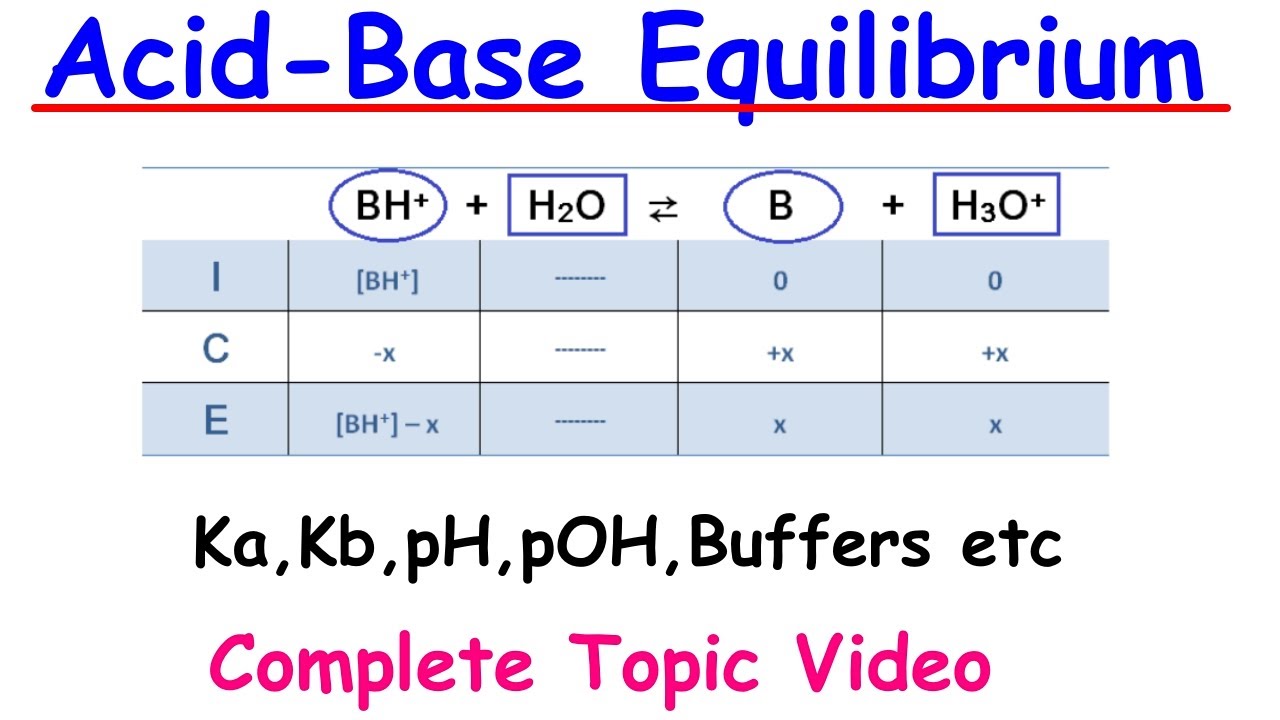
- 🧪 The dissociation of a weak base is represented by the KB expression, which involves the concentration of the base (B), its conjugate acid (HB+), and the hydroxide ion (OH-).
- 🤔 The difference between H+ and hydronium (H3O+) is important to understand, as it affects the expressions for KA and KB.
- 📈 KA is used for acids, while KB is used for bases, corresponding to their respective dissociation in water.
- 💡 Strong acids and bases completely dissociate in solution, making their equilibrium constants very large and product-favored.
- 🔄 The relationship between KA and KB can be found using the ion product of water (KW), which is equal to KA times KB.
- 🔢 If given a KA value, one can find the corresponding KB value (and vice versa) by using the KW relationship.
- 🎓 Understanding KA and KB is crucial for analyzing the equilibrium of weak acids and bases in chemistry.
- 📊 The script provides a comprehensive overview of when and how to use KA and KB in the context of acid-base chemistry.
Q & A
What are Ka and Kb used to describe in the context of acid-base equilibrium?
-Ka and Kb are equilibrium constants used to describe the dissociation of weak acids and weak bases in solution.
How are equilibrium constants defined?
-Equilibrium constants are defined as the ratio of the concentration of products to the concentration of reactants at equilibrium.
What is the relationship between H+ and the hydronium ion (H3O+)?
-H+ represents a proton, while the hydronium ion (H3O+) is a product of the self-ionization of water. The Ka expression uses the concentration of H3O+ instead of H+.
How is the Ka expression derived for the dissociation of an acid?
-The Ka expression is derived by writing out the dissociation of an acid in solution and then taking the ratio of the concentration of the products (A- and H3O+) to the reactants (HA).
What is the Kb expression for a base and how is it related to its conjugate acid?
-The Kb expression for a base is given by the concentration of its conjugate acid (HB+) times the concentration of OH- over the concentration of the initial base (B). It describes the dissociation of the base in water.
When should you use Ka and when should you use Kb in calculations?
-Use the Ka expression when dealing with a weak acid and the Kb expression when dealing with a weak base. Strong acids and bases completely dissociate, so Ka and Kb are not typically used for them.
What is the significance of the value of Ka or Kb in terms of dissociation?
-A larger Ka or Kb value indicates a greater degree of dissociation for the weak acid or base, meaning the reaction favors the formation of products more strongly.
How can you find the Kb value if you know the Ka value for a corresponding conjugate acid?
-You can find the Kb value using the relationship kW = Ka * Kb, where kW is the ion product of water. By dividing kW by Ka, you can find Kb, and vice versa.
What is the ion product of water (kW) and how is it used in acid-base calculations?
-The ion product of water (kW) is the product of the concentrations of H+ and OH- in water at equilibrium. It is used to find either Ka or Kb when one is known, using the relationship kW = Ka * Kb.
Why are Ka and Kb expressions most useful for weak acids and bases?
-Ka and Kb expressions are most useful for weak acids and bases because their dissociation is reversible and not completely favored, unlike strong acids and bases which completely dissociate in solution.
How can the Ka and Kb values be used to understand the strength of an acid or a base?
-A higher Ka value indicates a stronger acid, as it dissociates more in solution. Similarly, a higher Kb value indicates a stronger base, as it too dissociates more in solution.
Outlines
📘 Introduction to Ka and Kb
This paragraph introduces the topic of Ka and Kb, emphasizing their importance in understanding acid-base equilibrium. The speaker, Sarin, defines Ka and Kb and explains their practical significance. It is highlighted that Ka and Kb are types of equilibrium constants, which are essentially the ratio of products to reactants. The concept of dissociation constants is introduced, explaining that Ka and Kb describe the degree of dissociation in weak acids and bases. The video also suggests watching a previous video on equilibrium constants for a better understanding, and the process of deriving Ka and Kb expressions from the dissociation of acids and bases in solution is outlined.
Mindmap
Keywords
💡acid-base equilibrium
💡Ka
💡Kb
💡equilibrium constants
💡dissociation constants
💡conjugate acid-base pairs
💡weak acids and bases
💡Ionization
💡hydronium and hydroxide ions
💡Kw
💡equilibrium expressions
💡chemical equilibrium
Highlights
Ka and Kb are important when discussing acid-base equilibrium.
Ka and Kb are equilibrium constants that describe the dissociation of weak acids or weak bases.
Equilibrium constants are a ratio of products to reactants.
Ka is used for acids, while Kb is used for bases.
Strong acids and bases completely dissociate in solution, making Ka and Kb very large for them.
Ka and Kb are particularly useful for discussing the equilibrium of weak acids or weak bases.
The Ka expression is derived from the dissociation of an acid in solution.
The Kb expression is derived from the dissociation of a base in water.
The Ka expression is given by the concentration of the conjugate base (A-) times the concentration of hydronium (H3O+) over the concentration of the acid (HA).
The Kb expression is given by the concentration of the conjugate acid (HB+) times the concentration of hydroxide (OH-) over the concentration of the base (B).
Ka and Kb can describe the amount of dissociation in weak acids or bases.
The difference between H+ and hydronium (H3O+) is explained in the video.
Professors may give a Ka value and ask you to find the corresponding Kb value for the conjugate base of an acid.
You can use the ion product of water (Kw) to find either Ka or Kb, given one value.
Kw equals Ka times Kb, which can be used to find the unknown constant.
The video provides practical insights into when and how to use Ka and Kb in acid-base equilibrium discussions.
Transcripts
Browse More Related Video

18.2 Acid and base dissociation constants Ka and Kb (HL)

pKa and pKb relationship | Acids and bases | Chemistry | Khan Academy

What's the Difference between Ka and Kb?

ALEKS - Predicting the Qualitative Acid-Base Properties of Salt
Acid Base Equilibrium Full Topic Video

16.5 pH Calculations for Weak Acids and Bases | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: