Intro to Balancing Chemical Equations
TLDRThis educational script delves into the fundamental concept of balancing chemical equations, emphasizing the importance of adhering to the law of conservation of mass. It illustrates the process of balancing through various examples, starting with simple reactions like the formation of water from hydrogen and oxygen molecules, and progressing to more complex ones involving fractions. The script clarifies misconceptions, such as the incorrect belief that balancing equations is related to energy conservation, and highlights the significance of using whole numbers or fractions to achieve a balanced state, ultimately simplifying the equations to their lowest whole number ratios.
Takeaways
- 🧪 Hydrogen and oxygen gases exist as diatomic molecules (H2 and O2) rather than single atoms because of their bonding preferences.
- 🔄 The formation of water (H2O) involves the combination of two hydrogen molecules (H2) with one oxygen molecule (O2), adhering to the law of conservation of mass.
- ⚖️ Balancing chemical equations is essential to ensure an equal number of atoms on both sides of the reaction, reflecting the reality that atoms are simply rearranged during chemical reactions.
- 📐 The law of conservation of mass states that in a chemical reaction, the total mass of the reactants equals the total mass of the products, with no loss or gain of atoms.
- 🔢 Balancing equations often starts with the most complex molecules or elements that appear multiple times, and coefficients are adjusted to achieve equality on both sides of the equation.
- 🚫 Changing subscripts in chemical formulas is not allowed during the balancing process, as it would alter the identity of the molecules.
- 🔍 When balancing equations, it's sometimes necessary to use fractions to achieve the correct ratio of atoms, which can later be cleared by multiplying through by a common factor.
- 🌐 The concept of chemical reactions involves the rearrangement of atoms to form new substances with different properties, demonstrating the variety that can be created from a limited number of elements.
- 🔬 In the historical context, the understanding of chemical reactions and the conservation laws has simplified the perception of countless unique substances into combinations of about 100 elements.
- 🌡️ The properties of substances can change dramatically with the rearrangement of atoms, as illustrated by the different states and properties of hydrogen and oxygen gases compared to liquid water.
- 📚 The script emphasizes the importance of practice in balancing chemical equations, highlighting it as a fundamental skill in chemistry.
Q & A
Why do hydrogen and oxygen atoms typically exist as diatomic molecules (H2 and O2) rather than as single atoms?
-Hydrogen and oxygen atoms typically exist as diatomic molecules because of the way they bond more stably in pairs. This is due to their valence electrons, which form a more stable configuration when they are paired up, allowing them to achieve a full outer shell and thus lower energy state.
What is the law of conservation of mass, and how does it apply to chemical reactions?
-The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. It implies that the total mass of the reactants equals the total mass of the products. In terms of atoms, this means that the number and type of atoms must remain constant before and after the reaction, only their arrangement changes.
Why can't we change the subscripts in a chemical formula when balancing equations?
-Changing the subscripts in a chemical formula would alter the identity of the molecule, creating a different substance. The subscripts indicate the number of atoms of each element in a molecule, and they must remain constant to maintain the molecular identity during the balancing process.
How can you use fractions when balancing chemical equations, and why is it necessary?
-Fractions can be used to balance chemical equations when the number of atoms of a particular element does not match between reactants and products. Fractions allow for the correct ratio of molecules to be determined, ensuring that the law of conservation of mass is upheld. It's necessary because sometimes whole number coefficients do not provide a balanced equation, and fractions can accurately represent the ratio needed.
What is the significance of balancing chemical equations in understanding chemical reactions?
-Balancing chemical equations is crucial for understanding the stoichiometry of a reaction, which is the quantitative relationship between the amounts of reactants and products. It ensures that the reaction adheres to the law of conservation of mass and provides a basis for predicting the outcomes and yields of chemical reactions.
How does the script illustrate the process of balancing the chemical equation for the formation of water (H2O)?
-The script demonstrates the process by initially presenting an unbalanced equation, H2 + O2 → H2O, and then progressively adjusting coefficients to ensure that the number of hydrogen and oxygen atoms on both sides of the equation are equal, resulting in the balanced equation 2H2 + O2 → 2H2O.
What is the difference between a balanced chemical equation and an unbalanced one?
-A balanced chemical equation has the same number of atoms for each element on both sides of the equation, complying with the law of conservation of mass. An unbalanced equation has an unequal number of atoms for one or more elements, which does not reflect a possible chemical reaction.
Why do CO2 and H2O form during most combustion reactions?
-CO2 and H2O form during most combustion reactions because they are stable molecules. Combustion typically involves the reaction of a fuel with oxygen, and the products tend to form the most stable configurations possible, which for many elements and compounds include carbon dioxide and water.
What is the purpose of including states of matter (solid, liquid, gas, aqueous) in a chemical equation?
-Including states of matter in a chemical equation provides information about the physical form of the reactants and products. This is important for understanding the conditions under which the reaction occurs and can affect the reaction rate and mechanism.
Can you explain the concept of 'coefficients' in the context of chemical equations?
-In chemical equations, coefficients are the numbers placed in front of the chemical formulas to indicate the number of molecules or formula units of a reactant or product involved in the reaction. They are used to balance the equation, ensuring that the number of atoms of each element is the same on both sides of the equation.
How does the script handle the introduction of fractions in balancing chemical equations, and why is it important to clear fractions afterward?
-The script introduces fractions as a tool to balance equations when whole number coefficients cannot achieve balance. It emphasizes that while fractions can be used during the balancing process, the final balanced equation should consist of whole numbers only. This is achieved by multiplying every term in the equation by the denominator of the fraction to clear the fractions, resulting in a simplified, whole-number balanced equation.
What are the steps to balance a complex chemical equation as demonstrated in the script?
-The script suggests starting with the most complex molecules first and leaving single atoms until the end. It also demonstrates using fractions to balance parts of the equation and then multiplying through by a common number to clear the fractions and achieve whole number coefficients.
Why is it important to balance the number of atoms in a chemical reaction?
-Balancing the number of atoms in a chemical reaction is important because it adheres to the law of conservation of mass, ensuring that atoms are neither created nor destroyed during the reaction. This principle is fundamental to understanding and accurately representing chemical reactions.
Outlines
🌊 Understanding Water Formation
This paragraph discusses the formation of water (H2O) from hydrogen and oxygen atoms. It explains that hydrogen and oxygen exist as diatomic molecules (H2 and O2) rather than single atoms. The speaker clarifies that water is formed through the combination of these diatomic molecules, specifically two hydrogen molecules (H2) and one oxygen molecule (O2), resulting in two water molecules (2H2O). The concept of a balanced chemical reaction is introduced, emphasizing the importance of the law of conservation of mass.
🔄 Balancing Chemical Reactions
The speaker delves into the process of balancing chemical reactions, using the example of water formation. They explain that coefficients (numbers placed in front of chemical formulas) can be adjusted to balance the number of atoms on both sides of the reaction. The paragraph highlights the importance of not altering the subscripts within the molecules, as these define the molecular identity. The law of conservation of mass is reiterated, emphasizing that the number of atoms must be the same before and after the reaction.
🔥 Methane Combustion Reaction
This paragraph introduces the combustion of methane (CH4) as another example of a chemical reaction. The speaker explains that methane reacts with oxygen (O2) to produce carbon dioxide (CO2) and water (H2O). The process of balancing this reaction is discussed, emphasizing the need to balance the number of hydrogen atoms first by adjusting the coefficient in front of the water molecule. The speaker also notes that CO2 and H2O are stable molecules, often resulting from combustion reactions.
🌐 States of Matter in Chemical Reactions
The speaker introduces the concept of including states of matter in chemical reactions, using the example of methane combustion. They explain that the states of matter (solid, liquid, gas, aqueous) are indicated by symbols (s, l, g, aq) and are crucial for understanding the physical context of the reaction. The paragraph also discusses the importance of writing balanced chemical reactions, ensuring that the number of atoms is the same on both sides.
🤔 Balancing Complex Reactions
The speaker tackles a more complex reaction involving cobalt (Co), carbon, and oxygen. They explain the process of balancing the reaction by focusing on the more complex molecules first and leaving the simpler elements (like single atoms) for last. The paragraph demonstrates the use of coefficients to balance the oxygen atoms and the importance of not changing the subscripts within the molecules. The speaker also emphasizes the need to balance the reaction in a step-by-step manner.
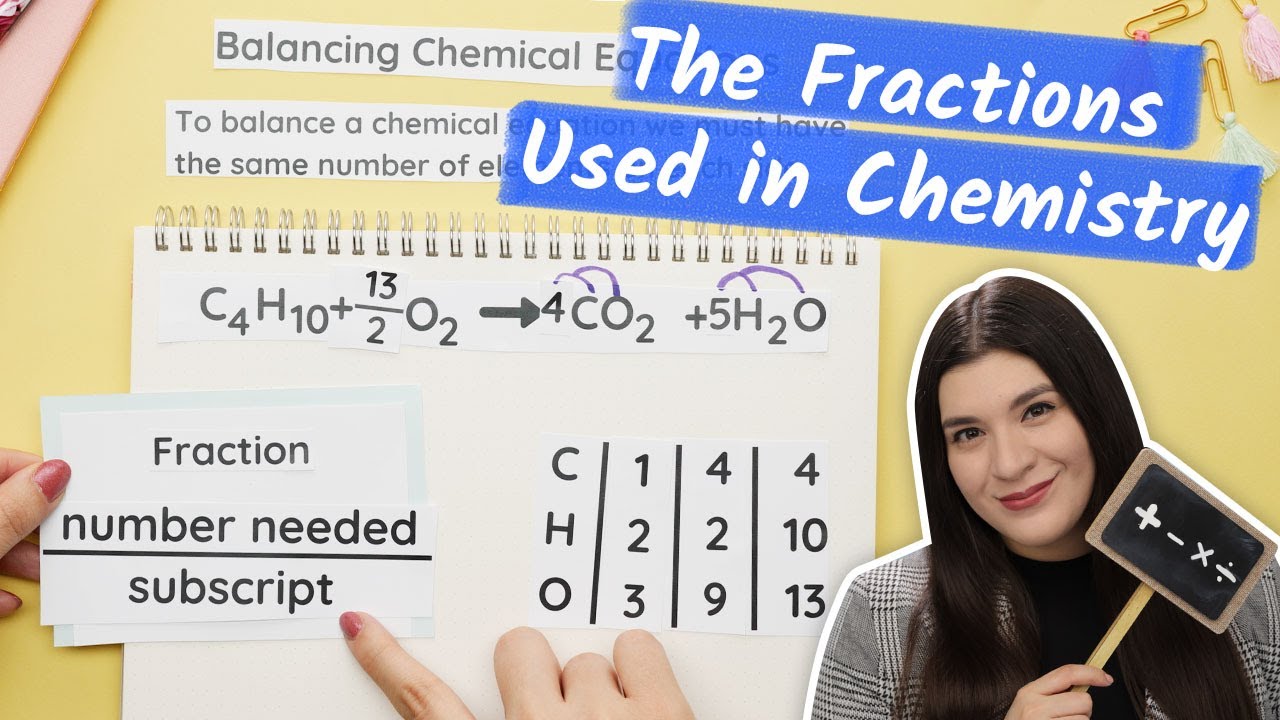
🔍 Detailed Balancing of Butane Reaction
This paragraph focuses on balancing the combustion reaction of butane (C4H10) with oxygen (O2) to form carbon dioxide (CO2) and water (H2O). The speaker explains the process of balancing carbon and hydrogen atoms first, then addresses the oxygen atoms. They introduce the concept of using fractions to balance the reaction and emphasize the need to clear fractions by multiplying through the entire equation to achieve whole numbers in the final balanced reaction.
📚 Rules of Thumb for Balancing Reactions
The speaker provides tips for balancing chemical reactions, suggesting that complex molecules should be addressed first, followed by simpler elements. They discuss the process of balancing a reaction involving cobalt oxide and carbon, demonstrating the use of fractions and the importance of clearing these fractions by multiplying through the equation. The paragraph also emphasizes the importance of writing reactions multiple times to avoid confusion and ensure accuracy.
🌐 Law of Conservation of Mass
This paragraph discusses the fundamental principle behind balancing chemical reactions: the law of conservation of mass. The speaker clarifies that chemical reactions are balanced to ensure that the number of atoms is the same on both sides of the reaction, not because of the law of conservation of energy. They also address common misconceptions and emphasize the importance of understanding that atoms are rearranged during reactions, not created or destroyed.
🔬 Simplifying Chemical Reactions
The speaker concludes by discussing the simplification of chemical reactions, using an example of generic molecules A and B. They explain that reactions can be simplified by dividing through common numbers to achieve the lowest whole number ratio. The paragraph emphasizes the importance of expressing chemical reactions in their simplest form, highlighting the breakthrough in understanding that all substances are made of a limited number of elements combined in different ways.
Mindmap
Keywords
💡Hydrogen
💡Oxygen
💡Diatomic Molecule
💡Chemical Reaction
💡Law of Conservation of Mass
💡Balancing Chemical Equations
💡Methane (CH4)
💡Carbon Dioxide (CO2)
💡States of Matter
💡Aqueous
💡Fractions in Chemical Equations
Highlights
Explanation of the necessity of hydrogen and oxygen to form water (H2O).
Concept of diatomic molecules where gases like hydrogen and oxygen exist as pairs, not single atoms.
Illustration of the bonding process between hydrogen and oxygen to form water molecules.
Introduction to the law of conservation of mass in chemical reactions.
Demonstration of how to balance a simple chemical reaction, H2 + O2 to form H2O.
Clarification on the improper initial attempt to balance the water formation reaction and the correction to it.
Explanation of the correct ratio for the reaction of hydrogen and oxygen to form water, 2H2 + O2 yields 2H2O.
Importance of using the lowest whole number coefficients in balanced chemical equations.
Introduction to the combustion reaction of methane (CH4) with oxygen (O2) to form carbon dioxide (CO2) and water (H2O).
Step-by-step balancing of the methane combustion reaction emphasizing the rules of thumb in balancing chemical equations.
Discussion on the stability of CO2 and H2O molecules as products of combustion reactions.
Introduction of the concept of states of matter in chemical reactions with examples of gas, solid, and aqueous states.
Balancing of a more complex chemical reaction involving cobalt, carbon, and oxygen.
Explanation of the use of fractions in balancing chemical equations and the subsequent clearance of fractions for whole number coefficients.
Balancing of the butane combustion reaction highlighting the use of fractions and multiplication to achieve whole number coefficients.
Final discussion on the importance of balancing chemical equations according to the law of conservation of mass.
Clarification of misconceptions regarding the law of conservation of energy versus mass in the context of balancing chemical equations.
Practical application of balancing chemical equations through true or false questions to reinforce understanding.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: