Passing Gases: Effusion, Diffusion, and the Velocity of a Gas - Crash Course Chemistry #16
TLDRThis video explains the concept of gas velocity, distinguishing between the net velocity of a gas sample and the average velocity of its individual particles. It explores how temperature, kinetic energy, particle mass and collisions affect velocity. It introduces key gas motion concepts like Graham's law of effusion, concentration gradients, and diffusion. To demonstrate these principles, it depicts an experiment observing diffusion rates of ammonia and hydrochloric acid gases. The script reviews central takeaways so viewers grasp core concepts about gas particle velocities and what influences them.
Takeaways
- 😃 The velocity of a gas refers to the speed and direction its particles move; net velocity describes overall motion while average velocity describes individual particles.
- 😖Calculating gas velocity is complex because particles move randomly, frequently colliding.
- 💡 Temperature connects to velocity via kinetic energy; hotter gases have faster moving particles.
- 🔬 Graham studied gas effusion rates, finding lighter gases pass through barriers faster, proportional to the square root of their molar mass.
- 📏 Graham's law compares effusion rates of different gases under identical conditions.
- 👃 Gases diffuse from high to low concentration until evenly distributed; concentration gradients guide this.
- 👥 Diffusion is random individual particle motion that gives the appearance of directed overall motion.
- ⚖️ Graham's law provides reasonably accurate diffusion rate estimates by ignoring particle collisions.
- 💨 Faster diffusing ammonia gas traveled ~3/5 through a tube while slower hydrochloric acid diffused ~2/5 before reacting.
- 🧪 Precipitation reactions can occur between gaseous reactants.
Q & A
What causes the rotten egg smell that is referenced in the beginning of the video?
-The rotten egg smell is caused by eggs producing hydrogen sulfide gas as they start to go bad. Hydrogen sulfide gas contains sulfur, which gives it a characteristic rotten egg odor.
What is the difference between the net velocity of a gas and the average velocity of its particles?
-The net velocity refers to the overall speed and direction at which a gas mass moves from one place to another. The average velocity refers to the statistical mean of the speeds of the individual gas particles, which may be moving in random directions.
What does Graham's Law of Effusion describe?
-Graham's Law of Effusion describes the relationship between a gas's rate of effusion through an opening and its molar mass. Specifically, the ratio of the rates of effusion of two gases is inversely proportional to the square roots of their molar masses.
How does temperature affect the velocity of gas particles?
-Increasing the temperature of a gas increases the average kinetic energy of its particles. Since velocity is proportional to the kinetic energy divided by mass, a temperature increase results in an increase in gas particle velocity.
What causes gases to exhibit diffusion?
-Gases diffuse from high concentration regions to low concentration regions due to random molecular collisions rather than purposive particle motion. This spreads the gas out until it is evenly dispersed.
What is a precipitation reaction?
-A precipitation reaction is a chemical reaction that results in the formation of an insoluble solid called the precipitate. In the video, ammonia and hydrochloric acid gases participate in a precipitation reaction to form solid ammonium chloride.
What does the rate of effusion measure?
-The rate of effusion measures the amount of gas (moles or volume) that passes through an opening per unit time rather than the distance traveled by the gas.
What causes lower net velocity of a gas compared to the average velocity of its particles?
-The collisions between fast and slow moving particles in a gas system hinder its overall motion, resulting in a lower net velocity compared to the high average velocity of the individual particles.
What is a concentration gradient?
-A concentration gradient refers to the difference in concentration between two points. Gases tend to move from regions of high concentration to low concentration.
How can Graham's Law be applied to diffusion?
-Graham's Law can provide reasonable estimates of diffusion behavior by ignoring intermolecular collisions and treating diffusion simply as effusion in all directions.
Outlines
😷 Intro to gases and how they travel
The first paragraph introduces the concept of gas velocity - the speed and direction gases move. It explains the difference between a gas's net velocity versus the average velocity of its individual particles. Factors affecting gas velocity like temperature, mass, and kinetic energy are mentioned.
🚀 Graham's Law relates a gas's rate of effusion to its mass
The second paragraph discusses Graham's Law of Effusion - how the rate at which a gas effuses through an opening relates to its mass. The paragraph shows sample calculations using the law and explains how it can be applied to diffusion.
💨 Gases diffuse from high to low concentration
The third paragraph explains diffusion - gases tend to move from high concentration regions to low concentration regions. It notes that particle collisions affect net gas velocity and discusses an experiment showing distances diffused by two gases are proportional to their masses.
Mindmap
Keywords
💡velocity
💡kinetic energy
💡effusion
💡diffusion
💡collisions
💡concentration gradient
💡molar mass
💡Graham's Law
💡precipitation reaction
💡ideal vs real gases
Highlights
Learn about gas velocity, key to understanding gas behavior
Net velocity of gas lower than average velocity of molecules
Gases move faster at higher temperatures due to increased kinetic energy
Velocity inversely proportional to square root of mass
Graham's law relates gas effusion rates to molar mass
Gases diffuse from high to low concentration
Concentration gradient like a hill gases roll down
Diffusion is random, not purposely directed
Graham's law estimates diffusion rates
Example showing ammonia diffusing faster than HCl
Precipitation reaction between gases observed
Distances gases traveled fit Graham's law prediction
Learned net vs average molecular velocity
Learned Graham's law and applying to effusion/diffusion
Gas particle collisions affect motion and calculations
Transcripts
Browse More Related Video
Kinetic Molecular Theory of Gases - Practice Problems
Kinetic Theory of Gases - A-level Physics
Gas Laws - Equations and Formulas
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion

9.3 Additional Gas Laws | Dalton's Law and Graham's Law | High School Chemistry
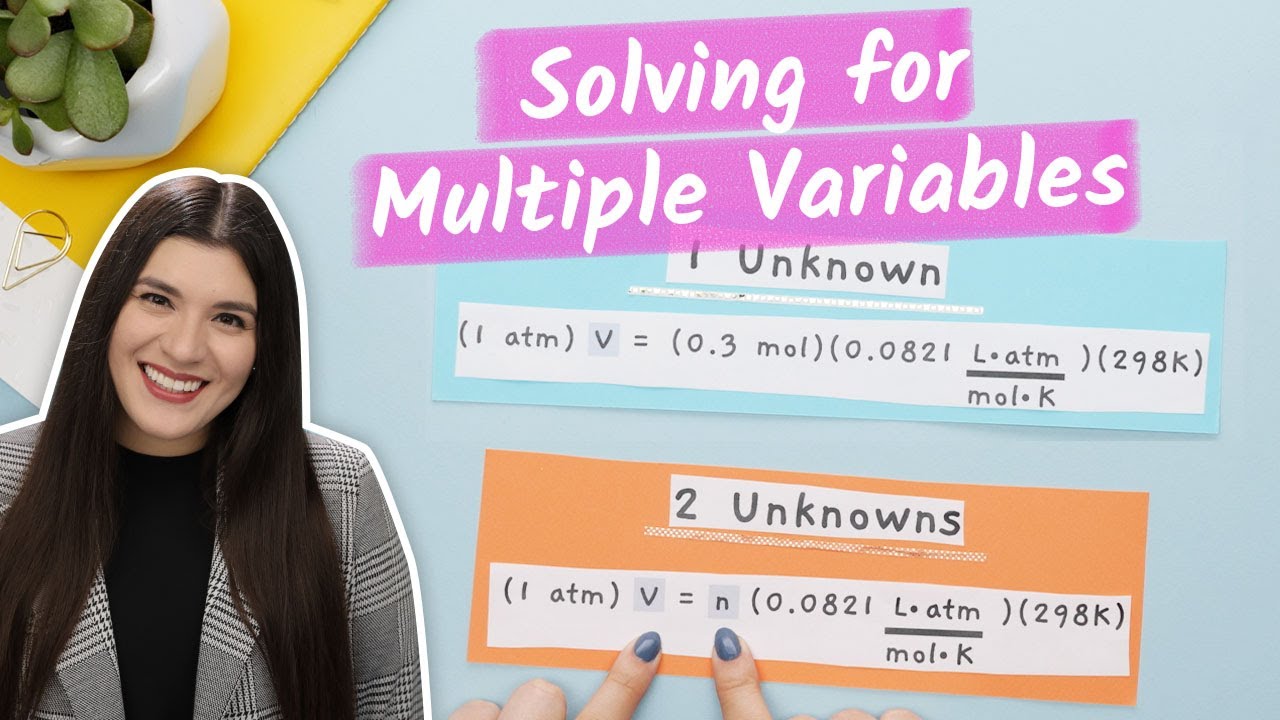
Solving Chemistry Problems With Multiple Variables
5.0 / 5 (0 votes)
Thanks for rating: