General Chemistry Review for Organic Chemistry
TLDRThis educational live stream delves into the foundational concepts of organic chemistry, focusing on chemical bonding and Lewis dot structures. The host guides viewers through drawing Lewis structures for atoms and molecules, emphasizing the importance of understanding valence electrons and covalent bonds. The discussion progresses to hybridization, molecular geometry, and the impact of electron pair repulsion on bond angles, using examples like methane, ammonia, and formaldehyde to illustrate key points. The session aims to equip viewers with a solid grasp of general chemistry principles essential for organic chemistry studies.
Takeaways
- 🧪 The script is from an educational live stream focused on organic chemistry, aiming to provide a foundational understanding before diving deeper into the subject.
- ⏳ The presenter expresses excitement for the upcoming stream, indicating the importance of the general chemistry concepts that will be covered for understanding organic chemistry.
- 🔬 The core of organic chemistry revolves around chemical bonding, especially since carbon can form various types of bonds, which is essential for the structure of organic compounds.
- 📚 Lewis dot structures are emphasized as a fundamental tool for depicting the connectivity between atoms in molecules and the arrangement of valence electrons.
- 👤 The presenter reminds viewers about the representation of atoms in Lewis dot structures, including how to denote the nucleus, core electrons, and valence electrons.
- 📈 The number of valence electrons determines an element's bonding behavior, with main group elements typically having a predictable number of bonds they can form.
- 🧩 The driving force behind bonding is the achievement of a stable electron configuration similar to that of noble gases, which is why elements form covalent bonds to fill their valence shells.
- 🔍 The script provides a step-by-step guide on how to draw Lewis dot structures for molecules, including counting valence electrons, arranging atoms, and distributing electrons to form bonds and lone pairs.
- 🌐 The difference between Lewis dot structures and Kekulé structures is highlighted, with the latter using lines to represent covalent bonds for simplicity.
- 📐 Valence bond theory and hybridization concepts are introduced to explain the geometry of molecules, including the formation of sigma and pi bonds, and the hybrid orbitals sp, sp2, and sp3.
- 📝 The process of predicting hybridization and geometry around a central atom involves identifying the central atom, counting sigma bonds and lone pairs, selecting the appropriate hybridization, and understanding the bond angles predicted by VSEPR theory.
Q & A
What is the main topic of the second episode of the organic chemistry live stream?
-The main topic of the second episode is the foundational concepts of general chemistry that are essential for understanding organic chemistry, with a focus on chemical bonding.
What is the significance of the clock ticking mentioned in the script?
-The clock ticking is a metaphor for the urgency and excitement to start learning chemistry, indicating that the time to begin is now and the enthusiasm for the subject is high.
What does the speaker find exciting about the upcoming live stream?
-The speaker is excited about the upcoming live stream because it covers fundamental concepts of chemical bonding that are crucial for a solid foundation in organic chemistry, and they feel more excited for this particular stream than for any other in a long time.
What is the core of organic chemistry according to the script?
-The core of organic chemistry is chemical bonding, which involves how carbon atoms form various types of bonds with other carbon and non-carbon atoms.
What is a Lewis dot structure and what information does it provide about a molecule?
-A Lewis dot structure is a graphical representation of a molecule that shows which atoms are connected to which, the arrangement of valence electrons, and the types of covalent bonds between atoms, such as single, double, or triple bonds.
How do main group elements determine the number of valence electrons, and is there an exception?
-Main group elements determine the number of valence electrons based on their group number in the periodic table. However, there is an exception with helium, which has two valence electrons despite being in the eighth group.
Why do non-metal elements form covalent bonds with other atoms?
-Non-metal elements form covalent bonds with other atoms to achieve a stable electron configuration similar to that of noble gases, by filling their valence shells with electrons through sharing.
What is the driving force behind the bonding behavior of non-metals as discussed in the script?
-The driving force behind the bonding behavior of non-metals is to achieve a noble gas electron configuration, which provides a stable and low potential energy state for the atoms.
What is the difference between a sigma bond and a pi bond in terms of orbital overlap?
-A sigma bond is a linear head-on overlap of orbitals, while a pi bond is a sideways overlap. Sigma bonds are typically formed in single covalent bonds, whereas pi bonds are part of double and triple covalent bonds.
What is the importance of hybridization in understanding molecular geometry?
-Hybridization is crucial for understanding molecular geometry because it explains how atomic orbitals mix to form hybrid orbitals that determine the shape and bond angles of molecules.
What are the three types of hybridizations involving s and p orbitals, and what do they result in?
-The three types of hybridizations involving s and p orbitals are sp3, sp2, and sp. sp3 hybridization results from combining one s orbital with three p orbitals, forming four sp3 hybrid orbitals. sp2 hybridization results from combining one s orbital with two p orbitals, forming three sp2 hybrid orbitals. sp hybridization results from combining one s orbital with one p orbital, forming two sp hybrid orbitals.
How does the presence of lone pairs affect the molecular geometry of a molecule?
-The presence of lone pairs affects the molecular geometry by altering the shape of the molecule. Lone pairs exert a repulsive force that influences the arrangement of atoms and the bond angles, leading to different geometries such as bent or trigonal pyramidal, depending on the hybridization and the number of lone pairs.
What is the approximate bond angle associated with sp3, sp2, and sp hybridization?
-The approximate bond angles associated with hybridizations are 109.5 degrees for sp3, 120 degrees for sp2, and 180 degrees for sp hybridization.
Can you provide an example of a molecule with sp3 hybridization and a tetrahedral molecular geometry?
-An example of a molecule with sp3 hybridization and a tetrahedral molecular geometry is methane (CH4), where the carbon atom is sp3 hybridized and bonded to four hydrogen atoms.
What is the process for predicting hybridization and molecular geometry around a central atom in a molecule?
-The process involves five steps: 1) Identify the central atom, 2) Count the number of sigma bonds and lone pairs around the central atom, 3) Select hybridization based on the number of orbitals required to hold electron pairs from step 2, 4) Understand that the approximate bond angles predicted by VSEPR theory are related to hybridization, and 5) Visualize the molecular shape using VSEPR theory, taking into account which electron pairs are bonding pairs and which are lone pairs.
How does the script differentiate between electron geometry and molecular geometry?
-Electron geometry refers to the arrangement of all electron pairs (both bonding and lone pairs) around an atom, while molecular geometry specifically refers to the arrangement of the atoms in a molecule, which can be affected by the presence of lone pairs.
What is the significance of sigma and pi bonds in understanding the properties of molecules?
-Sigma and pi bonds are significant because they determine the strength, stability, and reactivity of molecules. Triple bonds, which consist of one sigma and two pi bonds, are generally stronger and shorter than double bonds (one sigma and one pi), which in turn are stronger and shorter than single bonds (one sigma).
What is the approximate bond angle in a molecule with sp hybridization?
-The approximate bond angle in a molecule with sp hybridization is 180 degrees, resulting in a linear geometry.
What is the approximate bond angle associated with trigonal planar geometry?
-The approximate bond angle associated with trigonal planar geometry is 120 degrees.
How many valence electrons are there in a molecule with the formula C2H4?
-In a molecule with the formula C2H4, there are a total of 12 valence electrons (8 from the two carbon atoms and 4 from the four hydrogen atoms).
What is the central atom in the molecule with the formula C2H6?
-In the molecule with the formula C2H6, the central atoms are the two carbon atoms, as they are bonded to three hydrogen atoms each and are connected to each other.
What is the hybridization of the carbon atoms in the molecule with the formula C2H2?
-The hybridization of the carbon atoms in the molecule with the formula C2H2 (acetylene) is sp hybridization.
What is the approximate bond angle in a molecule with sp3 hybridization?
-The approximate bond angle in a molecule with sp3 hybridization is 109.5 degrees.
What is the significance of the lone pairs and bonding pairs in determining the molecular geometry?
-Lone pairs and bonding pairs are significant in determining molecular geometry because they affect the shape and bond angles of a molecule. Lone pairs exert a repulsive force that influences the arrangement of atoms, leading to different geometries based on the number of lone and bonding pairs present.
What is the next topic to be discussed in the organic chemistry live stream series?
-The next topics to be discussed in the organic chemistry live stream series include electronegativity and bond polarity, formal charge, resonance, and acid-base reactions and behavior of organic molecules.
Outlines
📚 Introduction to Organic Chemistry and Bonding Basics
The script begins with an energetic introduction to a live stream focused on organic chemistry, emphasizing the importance of a solid foundation in general chemistry concepts. It highlights the significance of chemical bonding as the core of organic chemistry, which is the study of carbon-containing compounds. The instructor expresses excitement for the topic and introduces the concept of Lewis dot structures as a fundamental tool for understanding molecular connectivity and covalent bonds. The summary also touches on the representation of atoms and their valence electrons in Lewis structures, setting the stage for a deeper dive into chemical bonding.
🔍 Understanding Valence Electrons and Predictable Bonding Behavior
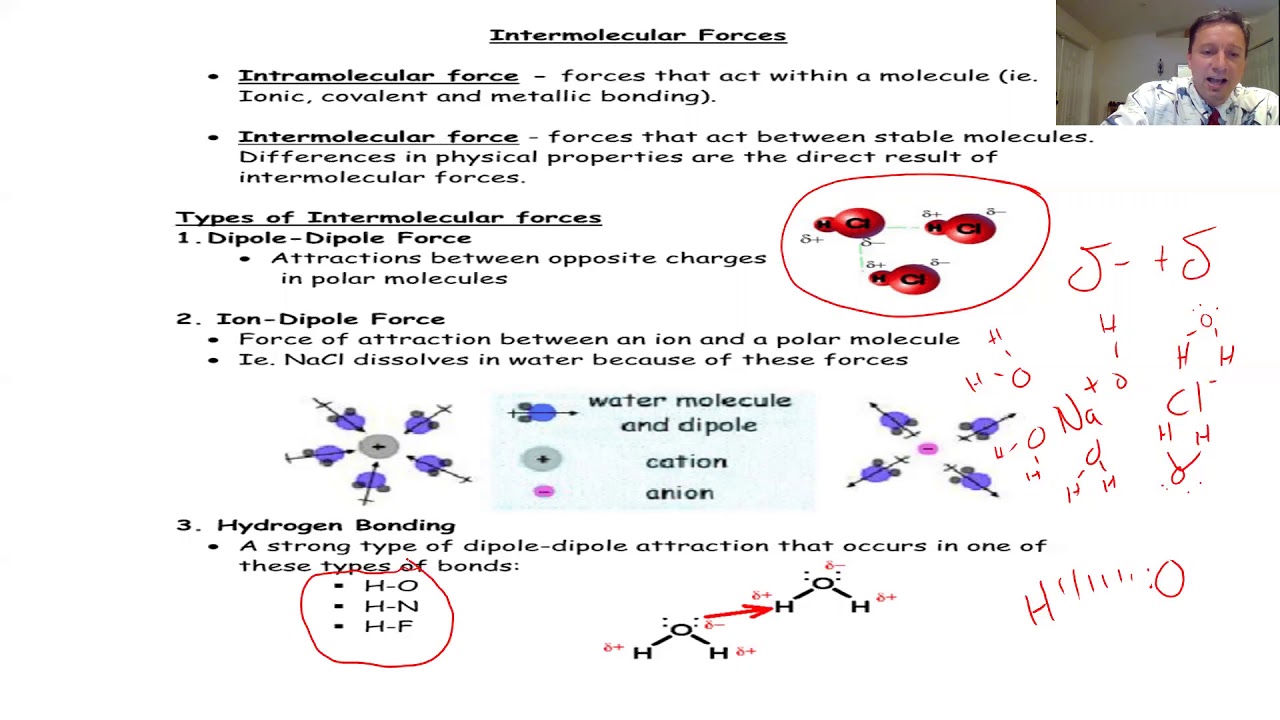
This paragraph delves into the specifics of valence electrons and their role in determining the predictable bonding behavior of main group elements. It explains how the number of valence electrons corresponds to the group number for most elements, with the exception of helium. The script uses common elements found in organic chemistry, such as carbon, nitrogen, oxygen, hydrogen, and halogens, to illustrate their bonding tendencies. The concept of noble gas electron configurations as a driving force for bonding is introduced, with examples provided to show how atoms aim to achieve stability akin to noble gases through covalent bonding.
📘 Steps to Draw Lewis Dot Structures for Molecules
The script outlines a step-by-step process for drawing Lewis dot structures for molecules, starting with counting total valence electrons and considering the charge of the molecule. It emphasizes the importance of identifying the central atom based on electronegativity and the inability of hydrogen to be a central atom due to its single bonding capacity. The steps continue with placing single covalent bonds, adding lone pairs starting from terminal atoms, and adjusting for an octet on all atoms, including the conversion of lone pairs to double or triple bonds where necessary. An example of drawing the Lewis structure for the nitrite ion is provided to illustrate the process.
🌐 Clarifying the Limitations of Lewis Structures in Representing Molecular Geometry
This section addresses the common misconception that Lewis structures can accurately represent the three-dimensional geometry of molecules. Using methane as an example, the script clarifies that while the Lewis structure of methane might suggest a planar configuration, the actual molecular shape is tetrahedral. The explanation includes a brief discussion on how to modify Lewis structures to better convey three-dimensional arrangements, emphasizing the limitations and the need for understanding actual molecular geometries separately from connectivity.
🔬 Valence Bond Theory and the Nature of Sigma and Pi Bonds
The script introduces Valence Bond Theory, which describes the formation of covalent bonds through the overlap of atomic orbitals. It differentiates between sigma and pi bonds, explaining that sigma bonds result from a head-on overlap of orbitals, while pi bonds are formed by a side-to-side overlap. Examples of sigma bonds are given for diatomic hydrogen and fluorine, as well as a heteronuclear sigma bond in hydrogen chloride. The explanation of pi bonds uses diatomic oxygen as an example, highlighting the distinction between the two types of bonds within a double covalent bond.
🌀 Hybridization and Its Impact on Molecular Geometry
This paragraph explores the concept of hybridization, which is the mixing of atomic orbitals to form hybrid orbitals that influence the geometry of molecules. It explains the principles behind hybridization, including the lowering of potential energy through bonding and the formation of hybrid orbitals reflecting the types and numbers of atomic orbitals involved. The script discusses the three types of hybridization involving s and p orbitals: sp3, sp2, and sp, each associated with different molecular geometries such as tetrahedral, trigonal planar, and linear. The importance of valence shell electron pair repulsion (VSEPR) theory in predicting molecular shapes is also highlighted.
🏗️ Building an Understanding of Molecular Geometries through Hybridization Examples
The script provides a detailed examination of the different types of hybridization, using specific examples to illustrate the resulting molecular geometries. It discusses sp3 hybridization in molecules like methane, where all hybrid orbitals are involved in bonding, resulting in a tetrahedral geometry. Other examples include sp2 hybridization in formaldehyde, leading to a trigonal planar geometry, and sp hybridization in hydrogen cyanide, which is linear. The explanation also covers the impact of lone pairs on molecular geometry, as seen in bent shapes for water and sulfur dioxide molecules.
🛠️ Predicting Hybridization and Geometry in Molecules
This section outlines a step-by-step method for predicting the hybridization and geometry around a central atom in a molecule. It emphasizes the importance of starting with the correct Lewis or Kekulé structure and identifying the central atom. The process involves counting sigma bonds and lone pairs, selecting the appropriate hybridization based on the number of orbitals required, understanding the bond angles predicted by VSEPR theory, and visualizing the molecular shape. The script encourages practice with molecules like C2H4 to reinforce the understanding of these concepts.
🔬 Analyzing the Hybridization and Geometry of Various Molecules
The script continues the discussion on hybridization and geometry with examples of different molecules, including C2H6, C2H2, and NH4+. Each example is used to demonstrate the process of determining the number of sigma bonds and lone pairs, selecting the appropriate hybridization (sp3, sp2, or sp), and identifying the resulting molecular geometry and bond angles. The analysis reinforces the principles introduced earlier and provides practical applications to various chemical structures.
🚀 Wrapping Up and Previewing Future Topics in Organic Chemistry
In the concluding part of the script, the instructor wraps up the current session by summarizing the key points covered on hybridization, molecular geometry, and the predictive process for these aspects of molecular structure. They also provide a preview of the topics to be covered in the next episode of the organic chemistry series, which will include electronegativity, bond polarity, formal charge, resonance, and acid-base reactions in organic molecules. The instructor expresses excitement for the upcoming sessions and thanks the audience for their participation.
Mindmap
Keywords
💡Chemical Bonding
💡Lewis Dot Structures
💡Valence Electrons
💡Noble Gas Electron Configuration
💡Covalent Bonds
💡Hybridization
💡Molecular Geometry
💡Sigma and Pi Bonds
💡Valence Shell Electron Pair Repulsion (VSEPR) Theory
💡Electronegativity
💡Resonance
Highlights
Introduction to the second episode of the organic chemistry live stream emphasizing the importance of a strong foundation in general chemistry.
Excitement for the upcoming stream is expressed, indicating the presenter's enthusiasm for the topic.
Chemical bonding is identified as the core concept of organic chemistry, focusing on carbon's ability to form various bonds.
Lewis dot structures are discussed as a fundamental tool for understanding molecular connectivity and valence electrons.
The procedure for drawing Lewis dot structures for individual atoms is reviewed, emphasizing the representation of valence electrons.
The relationship between an element's group number and its valence electrons is explained, with exceptions noted for elements like helium.
Predictable bonding behavior of main group elements is discussed, including the number of bonds elements like carbon, nitrogen, and oxygen typically form.
The concept of noble gas electron configuration is introduced as the driving force behind the formation of covalent bonds.
Examples of diatomic molecules and their bonding to achieve stable electron configurations are provided.
A step-by-step guide to drawing Lewis structures for molecules is presented, including counting valence electrons and arranging atoms.
The difference between Lewis and Kekulé structures is highlighted, with a preference for the cleaner appearance of Kekulé structures.
The limitations of Lewis and Kekulé structures in representing the three-dimensional geometry of molecules are discussed.
Valence bond theory is introduced, explaining sigma and pi bonds through the overlap of orbitals.
Hybridization concepts are explored, detailing how atomic orbitals mix to form hybrid orbitals that influence molecular geometry.
The principles of hybridization are connected to the stability of atoms and the formation of more stable covalent bonds.
Different types of hybridization (sp3, sp2, sp) are related to specific molecular geometries (tetrahedral, trigonal planar, linear).
A method for predicting hybridization and geometry around a central atom in a molecule is outlined, using a step-by-step approach.
Examples of determining hybridization and geometry for various molecules, such as C2H4, C2H6, and others, are demonstrated.
The significance of understanding electron geometry and molecular geometry in organic chemistry is emphasized for predicting molecular shapes.
A preview of topics for the next episode is given, including electronegativity, bond polarity, formal charge, resonance, and acid-base reactions.
Transcripts
Browse More Related Video

1.3 Valence Bond Theory and Hybridization | Organic Chemistry
AP Chemistry Unit 2 Review

AP Chemistry Unit 2 Review: Compound Structure and Properties (includes dot structure stuff :D)
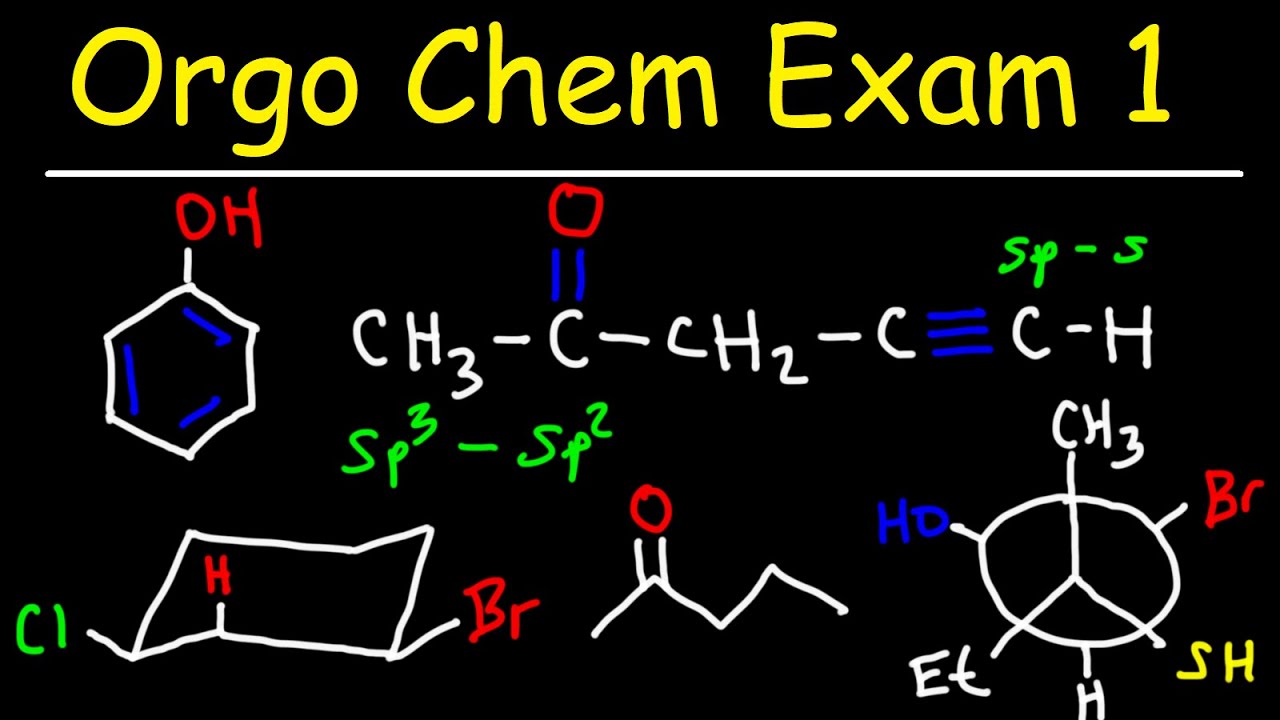
Organic Chemistry Exam 1 - IUPAC Nomenclature, Resonance, Acids & Bases, Newman Projections

1.1 Lewis Structures | Organic Chemistry Complete Course

8.2 How to Draw Lewis Dot Structures | Complete Guide | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: