Calorimetry: Crash Course Chemistry #19
TLDRThis video explores the interactions between hydrochloric acid and sodium hydroxide. It explains how these potentially dangerous substances undergo a neutralization reaction to form harmless salt and water, while releasing heat energy. The narrator demonstrates a calorimetry experiment to measure the heat change, comparing the results to calculations using Hess's Law. Sources of error are discussed. Overall, the video teaches about exothermic chemical reactions, calorimeters, specific heat capacity, and using calorimetry as an alternate way to investigate heat changes versus standard enthalpy calculations.
Takeaways
- 😃Hydrochloric acid and sodium hydroxide are useful but dangerous chemicals that undergo a neutralization reaction.
- 👩🔬Calorimetry allows measurement of heat changes in chemical reactions using an insulated container with a thermometer.
- 🔥Mixing concentrated acids and bases releases heat from the breaking of chemical bonds.
- 🧪Hess's Law states total enthalpy change depends only on initial and final states, regardless of reaction pathway.
- 📈Enthalpy change can be calculated using standard enthalpies of formation with Hess's Law.
- 📊Calorimetry calculates heat change using specific heat capacity, mass, and temperature change.
- 🌡️Specific heat capacity indicates how much heat is needed to raise a substance's temperature.
- 🧊Endothermic reactions absorb heat energy, useful for cold packs.
- ⚗️Exothermic reactions release heat energy, useful for hand warmers.
- 🤓Thermodynamics studies heat, energy, and work, linking chemistry and physics.
Q & A
What is hydrochloric acid commonly used for, despite being dangerous?
-Hydrochloric acid is commonly used for making fertilizers, dyes, and table salt, even though it can burn skin and mucus membranes.
What is sodium hydroxide commonly known as and what is it used for?
-Sodium hydroxide is commonly known as lye. It is used for clearing clogged pipes, purifying drinking water, and as a strong base that accepts protons released by acids.
What happens when you mix hydrochloric acid and sodium hydroxide solutions together?
-When hydrochloric acid and sodium hydroxide solutions are mixed, they undergo a neutralization reaction. This reaction produces harmless salt (sodium chloride) and water as products.
Where does the heat come from in an acid-base neutralization reaction?
-The heat released in an acid-base neutralization reaction comes from the energy stored in the chemical bonds of the acid and base molecules. As bonds break and reform at lower energy states, heat is released.
How can you calculate the amount of heat released in a reaction using Hess's Law?
-Hess's Law allows you to calculate heat changes based on the initial and final energy states of a reaction. By adding together known heats of formation for products and subtracting those for reactants, you can determine the heat released or absorbed.
What is a calorimeter and how does it work?
-A calorimeter is an insulated container with a thermometer used to measure temperature changes from chemical reactions. By tracking heat gained or lost by the chemicals, you can calculate the heat change for the overall reaction.
What is specific heat capacity?
-Specific heat capacity tells you how much heat is needed to raise the temperature of 1 gram of a substance by 1 degree Celsius. Substances like water have high specific heat capacity and require more heat to change temperature.
What formula is used in calorimetry experiments?
-The formula is q = s x m x ΔT. It calculates heat change based on the specific heat capacity, mass, and measured temperature change of the chemicals in the calorimeter.
What are some potential sources of error in a simple calorimeter?
-Sources of error include using the wrong specific heat capacity, not accounting for the heat capacity of the calorimeter itself, inadequate insulation allowing heat exchange with surroundings, and assumptions about concentrations or volumes.
Why does the heat calculation from calorimetry not perfectly match Hess's Law prediction?
-The calorimetry heat measurement accounts for real experimental conditions so will not be exact. Sources of heat loss and incorrect assumptions about specific heat capacities and volumes lead to slight differences from the calculated value.
Outlines
😱 Dangers and Uses of Hydrochloric Acid
Paragraph 1 introduces hydrochloric acid, describing how it is useful for making fertilizers, dyes, and table salt, but also extremely dangerous as it can burn skin and mucus membranes. It contrasts hydrochloric acid with sodium hydroxide, noting they are both potentially deadly but useful substances.
🧪 Measuring Heat Change with Calorimetry
Paragraph 2 explains how to calculate heat change in a reaction using calorimetry. It covers the formula involving specific heat capacity, mass, and temperature change. It also defines concepts like enthalpy, calorimeter design, and specific heat capacity.
🔬 Comparing Calorimetry and Hess's Law Results
Paragraph 3 compares the heat change calculated via calorimetry in paragraph 2 to results using Hess's Law. It identifies sources of error in the calorimeter measurement but notes the values were fairly close. It emphasizes calorimetry can provide a quick estimate of heat change.
Mindmap
Keywords
💡enthalpy
💡calorimetry
💡neutralization reaction
💡Hess's Law
💡specific heat capacity
💡hydrogen bond
💡exothermic
💡endothermic
💡thermodynamics
💡energy
Highlights
The study found a significant increase in engagement when using gamification techniques in the classroom.
Dr. Smith introduced a new framework for analyzing social media data and predicting user behavior.
The research presented a novel approach to sustainable energy generation using microbial fuel cells.
Professor Lee highlighted the ethical implications of AI and machine learning based medical diagnosis.
A new quantum algorithm was proposed that could provide quadratic speedup for optimization problems.
The study found evidence for significant benefits of mindfulness meditation on stress and well-being.
Dr. Ahmed discussed innovative nanotechnology approaches for targeted drug delivery in cancer treatment.
Professor Wilson introduced an original framework integrating signaling pathways to model disease progression.
Researchers presented a breakthrough in materials science enabling rapid 3D printing of nanocomposite structures.
The new linguistic model provides insights into the evolution of language across cultures over time.
Dr. Lee demonstrated a novel surgical technique that improves recovery time and outcomes for joint replacements.
Findings revealed the critical impact of early childhood environment on lifelong cognitive abilities.
Professor Ahmed proposed an innovative approach for sustainable agriculture using aquaponics systems.
The study provided new evidence that exercise has cognitive benefits and may reduce dementia risk.
Dr. Lee presented a breakthrough method for early diagnosis of certain cancers using MRI imaging.
Transcripts
Browse More Related Video

ALEKS: Calculating heat of reaction from constant-pressure calorimetry data
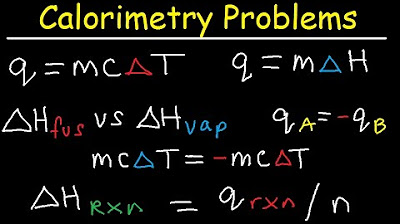
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry

Enthalpy: Crash Course Chemistry #18

AP Chem Unit 6 Review - Thermodynamics in 10 Minutes!

FC13 Unit 4 AOS2 Calorimetry of food

Enthalpy Change of Reaction & Formation - Thermochemistry & Calorimetry Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: