Enthalpy Change of Reaction & Formation - Thermochemistry & Calorimetry Practice Problems
TLDRThe video script delves into the calculation of enthalpy changes for various chemical reactions, focusing on combustion reactions as a primary example. It explains the process of balancing chemical equations and utilizes the concept of enthalpy of formation to determine the overall enthalpy change. The script introduces Hess's Law as a method for estimating enthalpy changes and demonstrates its application with different reactions, including the combustion of ethanol and ammonia's reaction with oxygen. Additionally, the script explores calorimetry as an alternative means to measure enthalpy changes, exemplified by the dissolution of sodium hydroxide and the heating of water to steam. It concludes with discussions on phase changes, such as melting, vaporization, and sublimation, and their respective enthalpy changes, providing a comprehensive understanding of thermal chemistry.
Takeaways
- 🔍 Balancing a chemical equation involves ensuring the number of atoms for each element is the same on both sides of the equation.
- 🔥 The enthalpy change of a reaction is calculated by summing the enthalpy of formation for products and subtracting the sum for reactants.
- ⚖️ Enthalpy of formation is the change in enthalpy when one mole of a substance is formed from its constituent elements.
- 🔄 Hess's Law allows for the calculation of enthalpy changes by combining known enthalpy changes of different reactions to find the enthalpy change of a reaction of interest.
- 📐 The specific heat capacity of a substance is the amount of heat required to raise the temperature of one gram of the substance by one degree Celsius.
- 🌡️ Calorimetry is a technique used to measure the enthalpy change of a reaction by observing the heat absorbed or released when a substance changes temperature.
- 🔊 The heat of fusion is the amount of energy required to change a substance from solid to liquid, and it is an endothermic process.
- 💧 Water has a high specific heat capacity, which means it can absorb a lot of heat before its temperature rises significantly.
- 🔥 Metals generally have low specific heat capacities, making them good conductors of heat but poor heat storers.
- 🧊 Phase changes, such as freezing, melting, vaporization, condensation, sublimation, and deposition, involve either the absorption or release of energy.
- ⚛️ The molar mass of a substance is the mass of one mole of that substance and is used to convert between grams and moles.
Q & A
What is the first step in balancing a combustion reaction?
-The first step in balancing a combustion reaction is to balance the carbon atoms first.
How is the enthalpy change of a reaction calculated using the enthalpy of formation values?
-The enthalpy change of a reaction is calculated by taking the sum of the enthalpy of formation for the products and subtracting the sum of the enthalpy of formation for the reactants.
What is the enthalpy of formation for a pure element in its natural state?
-The enthalpy of formation for a pure element in its natural state is zero.
How does one use Hess's Law to calculate the enthalpy change for a reaction?
-Hess's Law is used by adjusting and combining known reactions in such a way that they sum up to the target reaction, and then calculating the enthalpy change by adding the enthalpy changes of the individual reactions used.
What is the heat of fusion for water?
-The heat of fusion for water is about six kilojoules per mole, which is the energy required to convert ice at 0 degrees Celsius to liquid water at the same temperature.
What is the process called when a solid converts to a liquid?
-The process is called melting, and it is an endothermic process.
What is the heat capacity of water?
-The specific heat capacity of water is 4.184 joules per gram per degree Celsius.
How can you calculate the heat energy absorbed by water when sodium hydroxide is dissolved in it?
-You can calculate the heat energy absorbed by water using the formula Q = m*c*ΔT, where m is the mass of the water, c is the specific heat capacity of water, and ΔT is the change in temperature.
What is the enthalpy of combustion for benzene?
-The enthalpy of combustion for benzene is negative 3270 kilojoules per mole, indicating the amount of heat energy released when one mole of benzene is burned in air.
How do you calculate the amount of heat energy required to heat water from a lower temperature to steam at a higher temperature?
-The process involves multiple steps: heating the water to its boiling point, vaporizing the water to steam, and then heating the steam to the final temperature. Each step requires calculating the energy using the specific heat capacity for the temperature change and the heat of vaporization for the phase change.
Why do substances with a high specific heat capacity, like water, experience a smaller temperature change for the same amount of energy added compared to substances with a low specific heat capacity?
-Substances with a high specific heat capacity require more energy to raise their temperature, hence they can store more heat energy without a significant increase in temperature. In contrast, substances with a low specific heat capacity, like metals, experience a larger temperature change because they require less energy to increase in temperature and are better conductors of heat.
Outlines
🔍 Balancing Combustion Reactions and Calculating Enthalpy Change
This paragraph explains how to calculate the enthalpy change of a reaction, using the combustion of ethanol as an example. It details the steps to balance the chemical equation and then use the enthalpy of formation values for the products and reactants to determine the overall enthalpy change. The process involves summing the enthalpy of formation for the products and subtracting the sum for the reactants, leading to the enthalpy change of the reaction.
🔢 Understanding Enthalpy of Formation and Hess's Law
The paragraph delves into the concept of enthalpy of formation, which is the enthalpy change when one mole of a compound is produced from its elements in their natural state. It also introduces Hess's Law, which allows for the calculation of enthalpy changes for reactions that are not directly measurable by combining known enthalpy changes of other reactions. The explanation includes how to adjust and sum individual reactions to find the enthalpy change for a target reaction.
🧪 Application of Calorimetry in Enthalpy Change Calculations
This section illustrates the use of calorimetry to measure the enthalpy change of a reaction. It provides an example involving the dissolution of sodium hydroxide (NaOH) in water, where the temperature increase is used to calculate the heat absorbed by the water and subsequently the enthalpy change of the reaction. The calculation involves the use of the specific heat capacity of water and the temperature change to find the heat energy absorbed.
🔥 Enthalpy Change of Combustion and Phase Changes
The paragraph focuses on calculating the enthalpy change of combustion for benzene and the energy required for phase changes such as melting, vaporization, and sublimation. It explains how to convert between grams and kilojoules using thermochemical equations and discusses the concepts of endothermic and exothermic processes in the context of phase changes.
🌡️ Heat Energy Calculations for Temperature and Phase Changes
This part discusses how to calculate the energy required to heat water or ice through various temperature and phase changes. It covers the use of specific heat capacities and heat of fusion or vaporization in these calculations. The paragraph also explains how to break down complex problems into simpler parts and how to sum the energy changes for each part to get the total energy required for the process.
⚖️ Determining Specific Heat Capacity through Heat Transfer
The final paragraph presents a method to calculate the specific heat capacity of a metal using a heat transfer problem. It involves heating a metal and water mixture and finding the temperature at which they reach thermal equilibrium. By setting the heat lost by the metal equal to the heat gained by the water and using the specific heat capacity formula, the specific heat capacity of the metal can be determined.
Mindmap
Keywords
💡Enthalpy change
💡Combustion reaction
💡Hess's Law
💡Enthalpy of formation
💡Calorimetry
💡Phase changes
💡Heat capacity
💡Thermochemical equation
💡Endothermic process
💡Exothermic process
💡Molar mass
Highlights
The video focuses on calculating the enthalpy change of a reaction, specifically the combustion reaction of ethanol.
The process of balancing a combustion reaction is explained, emphasizing the order of balancing carbon, hydrogen, and oxygen atoms.
Enthalpy change of formation for substances such as ethanol, CO2, and H2O are introduced with specific values.
The formula for calculating the enthalpy change of a reaction using the enthalpy of formation is demonstrated.
An example calculation shows the enthalpy change of the ethanol combustion reaction to be -1366 kJ/mol.
The concept of enthalpy of formation as it relates to reactions where a compound is produced from its elements is discussed.
Hess's Law is introduced as a method for estimating the enthalpy change of a reaction by manipulating known reactions.
An example of using Hess's Law to calculate the enthalpy change for the combustion of ethanol is provided.
The calorimetry method is explained for estimating the enthalpy change of a reaction, using the dissolution of NaOH in water as an example.
The calculation of heat energy released during the combustion of benzene is demonstrated, using the enthalpy of combustion value.
Phase changes such as freezing, melting, vaporization, condensation, sublimation, and deposition are defined along with their enthalpy change signs.
The calculation of energy required to heat water from liquid to steam is shown, incorporating temperature change and phase change considerations.
The specific heat capacity of a metal is calculated using a heat transfer problem involving water and metal at different temperatures.
The significance of specific heat capacity in determining the temperature change of substances upon heat absorption or release is explained.
The difference between substances with high and low heat capacities, and their implications for heat storage and conduction, is discussed.
Practical applications of understanding enthalpy changes and specific heat capacities are highlighted in the context of chemical reactions and phase transitions.
Transcripts
Browse More Related Video
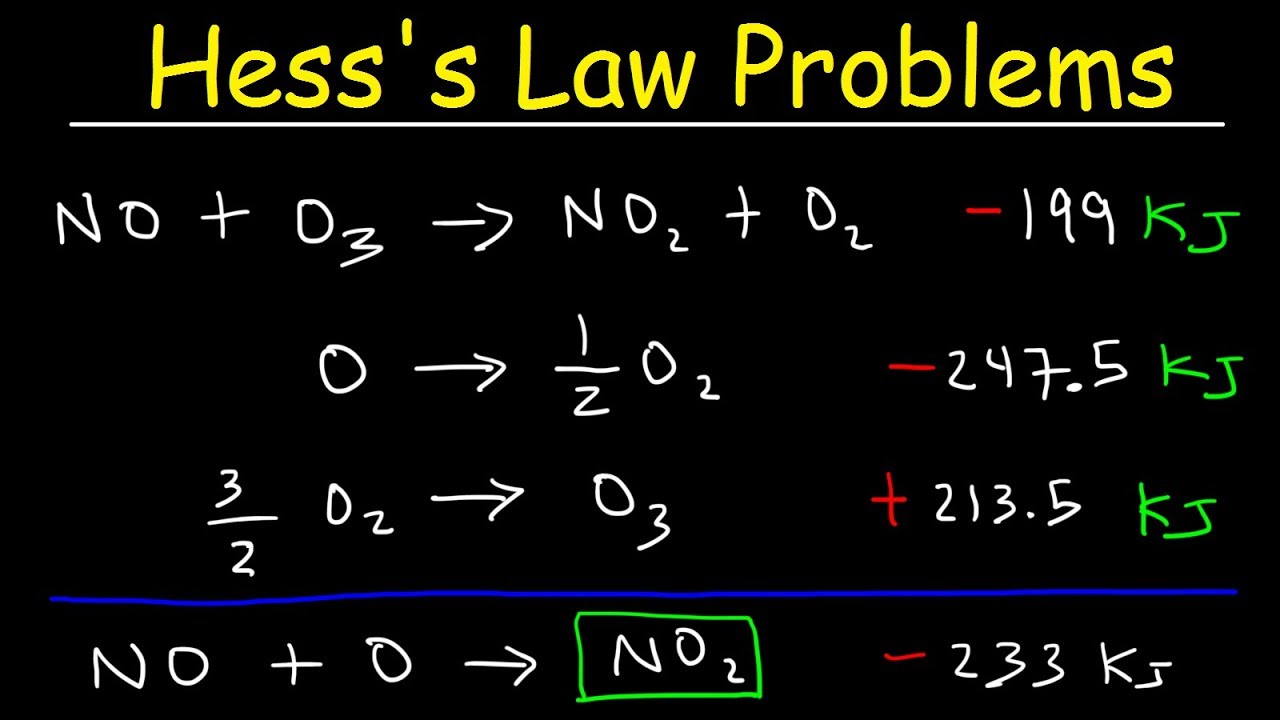
Hess's Law Problems & Enthalpy Change - Chemistry

Endothermic and Exothermic Reactions

Thermochemistry Equations & Formulas - Lecture Review & Practice Problems

Endothermic and Exothermic Reactions With Potential Energy Diagrams

Calorimetry: Crash Course Chemistry #19
Heat of formation | Thermodynamics | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: