FC13 Unit 4 AOS2 Calorimetry of food
TLDRThis chemistry video concludes a series on calorimetry, a technique to measure energy changes in substances. It revisits calorimetry principles, explains food calorimetry, and discusses its limitations like heat loss and incomplete combustion. The video introduces solution and bomb calorimeters for more accurate measurements and demonstrates how to calculate energy changes using the formula Q = mcΔT. It also covers calibration of calorimeters and thermochemical equations. The script includes practical examples and a multiple-choice question to test understanding, aiming to prepare students for exams.
Takeaways
- 🔍 Calorimetry is a method to measure the energy released or absorbed during a chemical reaction by observing the temperature change of a known amount of water.
- 📚 The formula Q = mcΔT is used to calculate the energy transferred, where Q is the heat energy, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
- 🌡️ In the context of food calorimetry, a piece of food is burned and the energy released is absorbed by water, but this method has limitations like heat loss and incomplete combustion.
- 🔢 The density of water is taken as 0.997 g/ml for more precise calculations instead of the commonly used 1 g/ml.
- 🔥 Bomb calorimeters are used for more accurate measurements, as they contain the reaction in a highly conductive cup (bomb), flush it with oxygen, and ignite it electronically to prevent heat loss.
- ⚗️ Solution calorimeters are used for reactions in solution, like exothermic redox reactions or dissolutions, and help reduce heat transfer to the environment by being insulated and covered.
- 🔄 Calorimeters can be calibrated by introducing a known amount of electrical energy and measuring the resulting temperature change in the water.
- 🔋 The calorimeter constant, or calibration factor, is determined by dividing the energy input (from electrical heating) by the temperature change observed.
- 🔢 Thermochemical equations can be written with a stated ΔH (enthalpy change) by using the energy change calculated from the temperature change and the number of moles involved in the reaction.
- ⚠️ Errors can be introduced in calorimetry experiments due to factors like incomplete combustion and heat loss to the environment, so precise equipment like bomb calorimeters is preferred.
- 📉 The heat of combustion can be determined by using the specific heat capacity of water and the temperature change observed after burning a sample in a calorimeter.
Q & A
What is calorimetry and how is it used in chemistry?
-Calorimetry is an experimental technique used to determine the amount of energy released or absorbed during a chemical reaction by measuring the temperature change of a known amount of water. It's used to calculate the energy transferred from a substance into the water using the formula Q = mcΔT, where Q is the heat energy, m is the mass of water, c is the specific heat capacity of water, and ΔT is the change in temperature.
What is the significance of the specific heat capacity of water in calorimetry?
-The specific heat capacity of water, which is approximately 4.18 joules per degree Celsius per gram, is crucial in calorimetry as it allows scientists to calculate the amount of heat energy (Q) transferred during a reaction. It's used in the formula Q = mcΔT to determine the energy changes in a system.
How does the calorimetry experiment account for incomplete combustion or heat loss?
-In the calorimetry experiment, issues like incomplete combustion and heat loss can introduce errors. To minimize these, more sophisticated calorimeters like solution calorimeters or bomb calorimeters are used. These devices are designed to reduce heat transfer to the environment and ensure more complete combustion, leading to more accurate measurements.
What is the difference between a solution calorimeter and a bomb calorimeter?
-A solution calorimeter is used for reactions that occur in solution and includes an insulated container with a thermometer to measure temperature changes. A bomb calorimeter, on the other hand, is a more advanced piece of equipment with a highly conductive cup (bomb) that contains the sample, which is then flushed with oxygen and ignited. This design minimizes heat loss and ensures complete combustion.
How can calorimeters be calibrated, and what is the purpose of this calibration?
-Calorimeters can be calibrated by introducing a known amount of electrical energy into the system and measuring the resulting temperature change. This process helps determine the calorimeter constant or calibration factor, which is the energy required to raise the temperature of the water by one degree. Once calibrated, the calorimeter can accurately measure the energy given off by a substance by monitoring temperature changes.
What is the formula used to calculate the energy (E) in a calorimeter calibration experiment?
-The formula used to calculate the energy (E) in a calorimeter calibration experiment is E = VIt, where V is the voltage, I is the current, and t is the time the current is applied in seconds.
How many significant figures should be reported in the results of a calorimetry experiment?
-The number of significant figures reported in the results of a calorimetry experiment should match those given in the original data. Typically, this would be three significant figures, as seen in the example provided in the script.
What is the purpose of determining the heat of combustion of charcoal in a bomb calorimeter?
-Determining the heat of combustion of charcoal in a bomb calorimeter allows for the measurement of the energy content of the charcoal. This is useful for understanding the energy released during combustion and can be expressed in kilojoules per gram.
How can the thermochemical equation for the dissolution of calcium chloride be written based on the information provided in the script?
-The thermochemical equation for the dissolution of calcium chloride can be written as: CaCl2 (s) → Ca^2+ (aq) + 2Cl^- (aq) with ΔH = -78 kJ/mol, indicating that the process is exothermic and releases 78 kilojoules of energy per mole of calcium chloride dissolved.
What is the importance of the calibration factor in determining the energy change for a reaction performed in a calorimeter?
-The calibration factor is essential as it relates the energy input to the temperature change observed in the calorimeter. By multiplying the calibration factor with the temperature change observed during a reaction, one can accurately determine the energy change for that reaction.
Outlines
🔍 Calorimetry and Energy Calculations
This paragraph introduces the concept of calorimetry, an experimental method used to measure the energy released from a substance by monitoring the temperature change of a water body. The formula Q = mcΔT is used to calculate the energy transferred, with specific values for the heat capacity of water and the mass of water calculated from its density. The paragraph also discusses the limitations of food calorimetry due to heat loss and incomplete combustion, and how these calculations can be applied to determine the energy required to raise the temperature of water from 18°C to boiling point, resulting in 222 kilojoules.
🔬 Advanced Calorimetry Techniques and Calibration
The second paragraph delves into more sophisticated calorimetry techniques, such as solution and bomb calorimeters, which are used to measure the energy released by substances more accurately. It explains the process of calibrating a calorimeter using a known amount of electrical energy and the temperature change it induces in water, leading to the determination of the calorimeter constant. The paragraph also outlines the steps for solving calorimetry problems, including calculating the energy change for a reaction and writing thermochemical equations with stated ΔH values. An example is provided where calcium chloride is dissolved in water, and the resulting temperature change is used to calculate ΔH.
🔢 Calorimetry Calculations and Thermochemical Equations
This paragraph continues the discussion on calorimetry, focusing on the calculation of the heat of combustion of charcoal using a bomb calorimeter. It emphasizes the importance of using the specific heat capacity of water and the mass of the substance to determine the energy content. The example provided calculates the heat of combustion as 20,407 joules per gram, converted to kilojoules for the final answer. The paragraph also touches on the importance of understanding the difference between energy content and enthalpy values.
📚 Calorimetry Practice and Multiple Choice Question
The final paragraph encourages viewers to practice calorimetry calculations with provided exam-style questions. It also presents a multiple-choice question involving the calibration of a calorimeter using an electric heating coil, where the goal is to determine the calorimeter's calibration factor in joules per degree Celsius. The correct answer is identified as 189 joules per degree Celsius, highlighting the importance of understanding and applying the formula E = VI*t to solve such problems.
Mindmap
Keywords
💡Calorimetry
💡Energy Transfer
💡Specific Heat Capacity
💡Food Calorimetry
💡Incomplete Combustion
💡Solution Calorimeter
💡Bomb Calorimeter
💡Calibration Factor
💡Thermochemical Equations
💡Significant Figures
Highlights
Introduction to the final content video on the journey through 3-4 chemistry.
Review of calorimetry, an experiment to determine energy released from substances by measuring temperature changes in water.
Explanation of the formula Q = mcΔT for calculating energy transferred, using 4.18 J/g°C as the specific heat capacity of water.
Clarification on using 0.997 g/ml instead of 1 g/ml for water density in calculations.
Introduction to food calorimetry experiments and the challenges of heat loss and incomplete combustion.
Calculation example to find the energy required to raise the temperature of 650 ml of water from 18°C to boiling.
Conversion of energy from joules to kilojoules for better representation with significant figures.
Discussion on the use of solution calorimeters and bomb calorimeters for more accurate energy measurements.
Description of a bomb calorimeter's setup and its advantages for minimizing heat transfer and ensuring complete combustion.
Process of calibrating calorimeters using known amounts of electrical energy and measuring temperature changes.
Explanation of the calorimeter constant or calibration factor and its role in determining energy given out by substances.
Use of the formula E = VI*t from Faraday's law to calculate energy in joules.
Step-by-step guide on solving calorimetry problems, including determining the calibration constant and energy change for reactions.
Example problem solving for the dissolution of calcium chloride, calculating ΔH and writing thermochemical equations.
A question on determining the heat of combustion of charcoal using a bomb calorimeter and the specific heat capacity of water.
Guidance on handling different ways information can be presented for calculating the calibration factor.
Multiple choice question to test understanding of calorimeter calibration using an electric heating coil.
Conclusion and encouragement to practice questions and prepare for exams.
Transcripts
Browse More Related Video

Calorimetry: Crash Course Chemistry #19

FC12 Unit 4 AOS2 Energy from food

Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics
2022 Live Review 6 | AP Chemistry | Thermodynamics Multiple-Choice and Free-Response Questions

6.2 Calorimetry | High School Chemistry
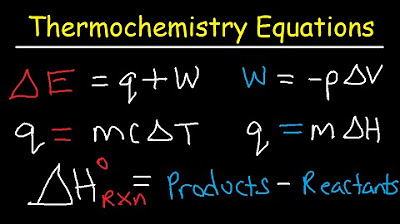
Thermochemistry Equations & Formulas - Lecture Review & Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: