AP Chem Unit 6 Review - Thermodynamics in 10 Minutes!
TLDRJeremy Krug's review of AP Chemistry Unit 6 focuses on the First Law of Thermodynamics, explaining the concepts of exothermic and endothermic processes and their effects on temperature. The video covers the formation of solutions and how it can be either exothermic or endothermic based on bond energy considerations. It introduces energy diagrams to visualize reaction energy changes and explains how to calculate heat transfer using the formula Q = mcΔT. The script also discusses specific heat capacity, phase changes, and their associated energy transfers. It concludes with methods to determine the enthalpy change of a reaction using bond enthalpies, standard enthalpies of formation, and Hess's Law. Krug invites viewers to join him for the next review on Chemical Equilibrium.
Takeaways
- 🔥 **Exothermic vs. Endothermic Processes**: Processes can either release (exothermic) or absorb (endothermic) energy, affecting the temperature of the surroundings.
- 🧲 **Energy Conservation**: In endothermic processes, the surroundings cool down, while in exothermic processes, they warm up, due to energy conservation.
- 💧 **Solution Formation**: The formation of a solution can be either exothermic or endothermic, depending on the energy of the bonds broken and formed with water molecules.
- 📈 **Energy Diagrams**: They illustrate the energy changes during a reaction, from reactants to products, including the transition state and activation energy.
- ✋ **Temperature and Kinetic Energy**: Average kinetic energy is directly related to temperature; heat is transferred from warmer to cooler materials until thermal equilibrium is reached.
- ♨️ **Heat Transfer Calculation**: The amount of heat transferred between systems can be calculated using the formula Q = mCΔT, where Q is heat, m is mass, C is specific heat capacity, and ΔT is temperature change.
- 🔆 **Specific Heat Capacity**: It measures a material's resistance to temperature change; materials with low C values heat up quickly, while those with high C values heat up slowly.
- 🌡️ **Heating Curves**: They show how temperature changes with added heat, with constant temperatures during phase changes like melting and boiling, indicating endothermic processes.
- 🔁 **Phase Changes**: Melting and boiling absorb energy (endothermic), while condensation and freezing release energy (exothermic), with opposite processes undoing each other.
- ⚖️ **Enthalpy of Reaction**: Chemical reactions are often accompanied by their enthalpy change (ΔH), which can be calculated using stoichiometry and given reaction data.
- 🔗 **Bond Enthalpies**: The change in enthalpy for a reaction can be determined by subtracting the total enthalpies of bonds formed from those broken, using a list of bond enthalpies.
- 📚 **Standard Enthalpies of Formation**: The change in enthalpy for a reaction is the sum of the enthalpies of formation of the products minus the reactants, with elements in their natural state having an enthalpy of 0 kJ/mol.
- 🔄 **Hess's Law**: If two reactions sum to form a new reaction, their ΔH values will sum to give the ΔH of the new reaction, allowing for manipulation of reactions to solve for unknowns.
Q & A
What is the First Law of Thermodynamics?
-The First Law of Thermodynamics, also known as the Law of Energy Conservation, states that energy cannot be created or destroyed in an isolated system. It can only be transferred or changed from one form to another.
What are the two types of processes in terms of energy transfer?
-The two types of processes are exothermic and endothermic. Exothermic processes involve the release of energy from the system to the surroundings, causing the surroundings to get warmer. Endothermic processes involve the system gaining energy from the surroundings, causing the surroundings to get colder.
Why might the formation of a solution be exothermic or endothermic?
-The formation of a solution can be exothermic or endothermic based on the energy of the bonds being broken and formed. If a high-energy bond is broken and a weaker bond with water molecules is formed, it's endothermic. Conversely, if a weak bond is broken and a stronger bond with water is formed, it's exothermic.
How do energy diagrams help in understanding a reaction's energy changes?
-Energy diagrams, or reaction coordinate diagrams, show the energy changes from reactants to products. They illustrate the activation energy needed to reach the transition state, and how the system's potential energy changes, indicating whether a reaction is exothermic (releases energy) or endothermic (absorbs energy).
What is the relationship between average kinetic energy and temperature?
-Average kinetic energy is directly proportional to temperature. As the temperature of a material increases, so does the average kinetic energy of its molecules, indicating more vigorous molecular motion.
How is heat transferred between two systems?
-Heat is transferred between two systems from the warmer object to the cooler one until thermal equilibrium is reached. This transfer occurs through molecular collisions and can be quantified using the equation Q = mcΔT, where Q is the heat transferred, m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
What does the equation Q = mcΔT represent?
-The equation Q = mcΔT represents the amount of heat transferred (Q) between two systems during a temperature change. It factors in the mass (m) of the substance, its specific heat capacity (c), and the change in temperature (ΔT).
How does the specific heat capacity of a material affect its temperature change?
-The specific heat capacity (c) is a measure of how much a material resists temperature change. Materials with low c values will experience large temperature changes with small amounts of heat, while those with high c values will have smaller temperature changes with the same amount of heat.
What are the characteristics of a heating curve?
-A heating curve shows how the temperature of a substance changes with the addition of heat. It illustrates that temperature remains constant during phase changes like melting and boiling (endothermic processes), and increases when heat is added to a solid, liquid, or gas.
What is the significance of enthalpy of reaction (ΔH) in a chemical equation?
-The enthalpy of reaction (ΔH) indicates the amount of heat absorbed or released during a chemical reaction. A negative ΔH signifies an exothermic reaction, where heat is released, while a positive ΔH indicates an endothermic reaction, where heat is absorbed.
How can bond enthalpies be used to calculate the enthalpy change (ΔH) of a reaction?
-Bond enthalpies can be used to calculate ΔH by subtracting the total enthalpy of bonds formed from the total enthalpy of bonds broken in the reaction. This method helps estimate the energy change during the making and breaking of chemical bonds.
What is Hess's Law and how is it applied in thermochemistry?
-Hess's Law states that the total enthalpy change for a chemical reaction is the same, no matter the path taken by the reaction. It is used to calculate the enthalpy change of a reaction by combining known enthalpy changes of other reactions that, when added together, result in the reaction of interest.
Outlines
🔥 First Law of Thermodynamics and Energy Transfer
Jeremy Krug introduces the AP Chemistry Unit 6 review focusing on the First Law of Thermodynamics. He explains the concepts of exothermic and endothermic processes, where energy is transferred between a system and its surroundings. The formation of a solution can be either exothermic or endothermic, depending on the bond energies involved. Energy diagrams are used to visualize these processes. The average kinetic energy, synonymous with temperature, is discussed in the context of thermal equilibrium and heat transfer. The formula for calculating heat transfer, Q = mCΔT, is presented, along with the concept of specific heat capacity. The conservation of energy and its implications for systems in contact are also covered. The heating curve and phase changes are explained, highlighting the endothermic nature of melting and boiling, and the exothermic nature of condensation and freezing. The relationship between enthalpy changes and chemical reactions is discussed, with examples provided for stoichiometry calculations.
⚗️ Calculating Enthalpy Changes in Reactions
This paragraph delves into methods for determining the change in enthalpy (ΔH) of a chemical reaction. It begins with the use of bond enthalpies to calculate ΔH by subtracting the total enthalpies of bonds formed from those broken. An example calculation is provided to illustrate this method. The concept of standard enthalpies of formation is introduced as another way to calculate ΔH, where the enthalpy change of a reaction is the sum of the enthalpies of formation of the products minus the sum for the reactants. The natural state of elements having an enthalpy of formation of zero is noted. Lastly, Hess's Law is explained as a method to find ΔH for a reaction by combining known ΔH values from other reactions, which may require manipulation such as flipping or multiplying reaction coefficients. An example demonstrates the process of combining reactions to find the overall ΔH. The paragraph concludes with a teaser for the upcoming Unit 7 review on Chemical Equilibrium.
Mindmap
Keywords
💡First Law of Thermodynamics
💡Endothermic process
💡Exothermic process
💡Energy diagrams
💡Thermal equilibrium
💡Heat transfer
💡Specific heat capacity
💡Enthalpy of reaction
💡Bond enthalpies
💡Standard enthalpies of formation
💡Hess's Law
💡Phase change
Highlights
The First Law of Thermodynamics is covered in AP Chemistry Unit 6.
Endothermic processes involve the system gaining energy from the surroundings, causing the surroundings to get colder.
Exothermic processes involve the system losing energy to the surroundings, causing the surroundings to get warmer.
The formation of a solution can be either exothermic or endothermic, depending on the bond energies involved.
Energy diagrams help visualize the energy changes during a reaction, from reactants to products.
Net loss of potential energy results in an exothermic reaction, while a net gain results in an endothermic reaction.
Average kinetic energy is synonymous with temperature, with higher temperatures indicating more kinetic energy.
Heat transfer occurs from warmer to cooler objects until thermal equilibrium is reached.
The amount of heat transferred between systems can be calculated using the equation Q = mcΔT.
Specific heat capacity measures a material's resistance to temperature change.
Heat gained by one system is equal to the heat lost by another, following the conservation of energy.
Heating curves illustrate how temperature changes with added heat, with phase changes showing constant temperature.
Melting and boiling are endothermic processes, while condensation and freezing are exothermic.
Opposite processes like freezing and melting, or boiling and condensation, undo each other's energy changes.
Chemical reactions can be accompanied by their enthalpy of reaction, denoted as ΔH.
Reaction stoichiometry can be used to calculate the energy released when forming a specific amount of product.
Bond enthalpies can be used to determine the change in enthalpy of a reaction by subtracting the energy of bonds formed from those broken.
Standard enthalpies of formation can be used to calculate ΔH by subtracting the sum of reactants' enthalpies from the sum of products' enthalpies.
Hess's Law states that the sum of two reactions' ΔH values equals the ΔH of the overall reaction when combined.
Jeremy Krug will cover Chemical Equilibrium in the next review of AP Chemistry Unit 7.
Transcripts
Browse More Related Video

8.3 Bond Enthalpy | High School Chemistry

Endothermic and Exothermic Reactions
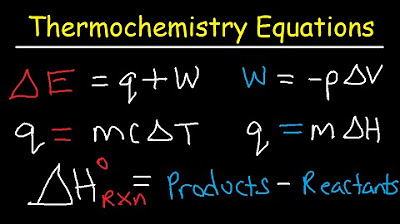
Thermochemistry Equations & Formulas - Lecture Review & Practice Problems

6.1 Reaction Enthalpy and Bond Dissociation Energy | Organic Chemistry

15. Thermodynamics: Bond and Reaction Enthalpies
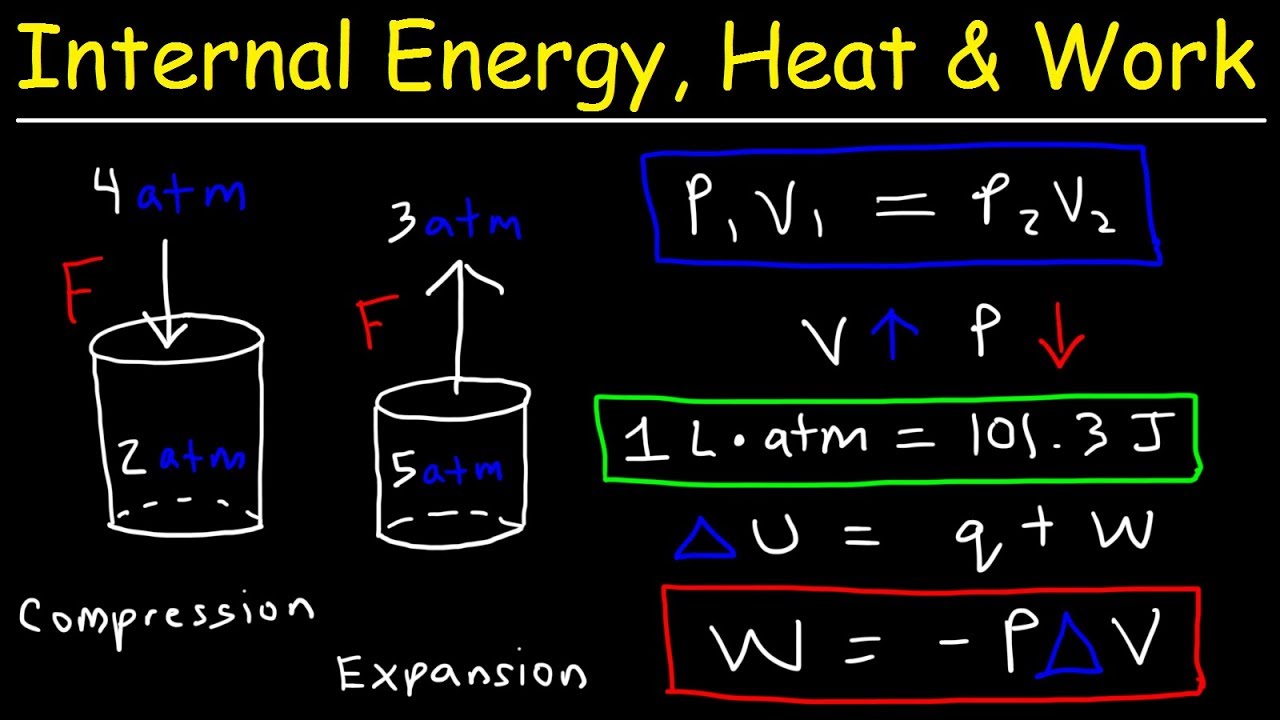
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
5.0 / 5 (0 votes)
Thanks for rating: