Thermochemistry Equations & Formulas - Lecture Review & Practice Problems
TLDRThis educational video delves into the principles of thermal chemistry, focusing on key concepts and equations essential for understanding the subject. It begins by explaining the internal energy of a system, detailing how it is affected by heat (q) and work (w). The video clarifies that heat transfer is from hot to cold, making q negative when heat leaves the system and positive when absorbed by the surroundings. It also covers the calculation of work done on or by a system, emphasizing the relationship between work, pressure, and volume change. A practice problem guides viewers through calculating the change in internal energy for a gas expanding at a specific pressure and volume change. The video further explores the heat energy calculation using the formula q = mcΔT, highlighting the specific heat capacity of water and how to calculate the energy required for temperature changes. It also touches on phase changes and the use of enthalpy of fusion or vaporization. The concept of thermal chemical equations is introduced with an example of propane combustion, demonstrating how to balance the reaction and calculate the heat energy released. The video concludes with an explanation of enthalpy of formation and how to estimate the enthalpy of a reaction using Hess's Law, providing a comprehensive understanding of thermal chemistry.
Takeaways
- 🔥 The change in internal energy of a system is equal to the heat energy added (q) plus the work done (w).
- ♨️ Heat flow is from hot to cold, making q negative when heat leaves the system and positive when absorbed by the surroundings.
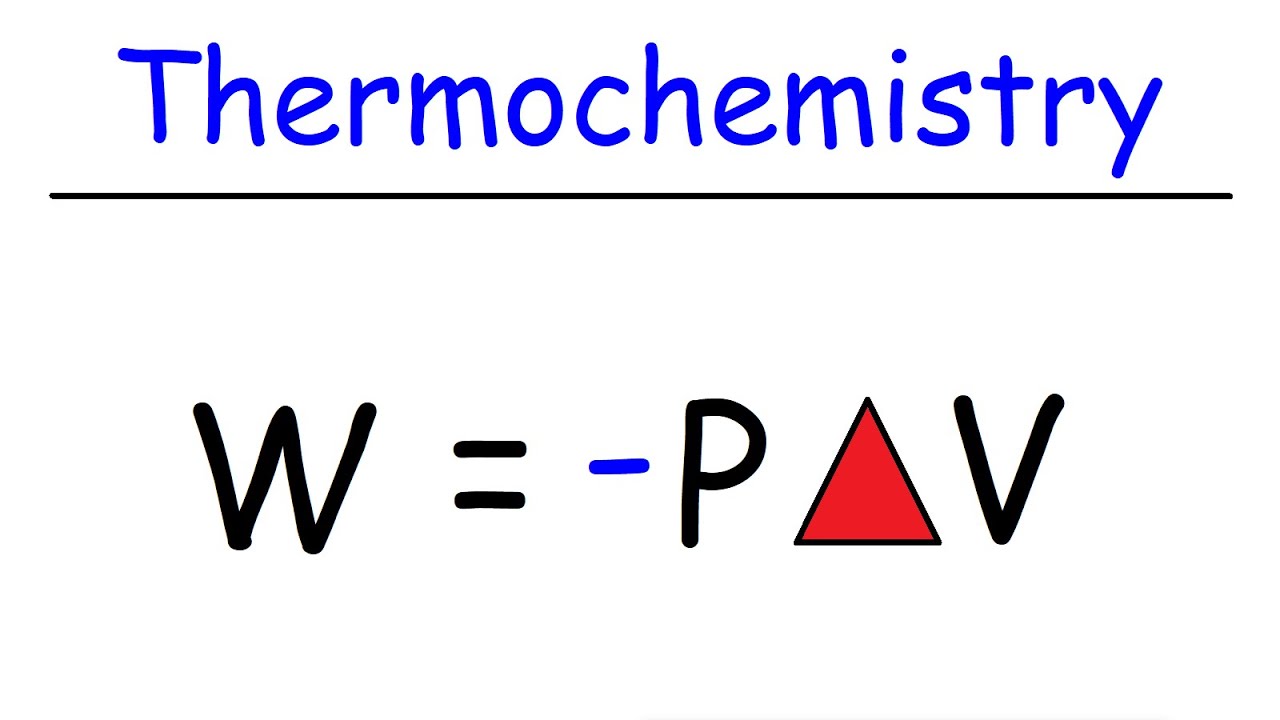
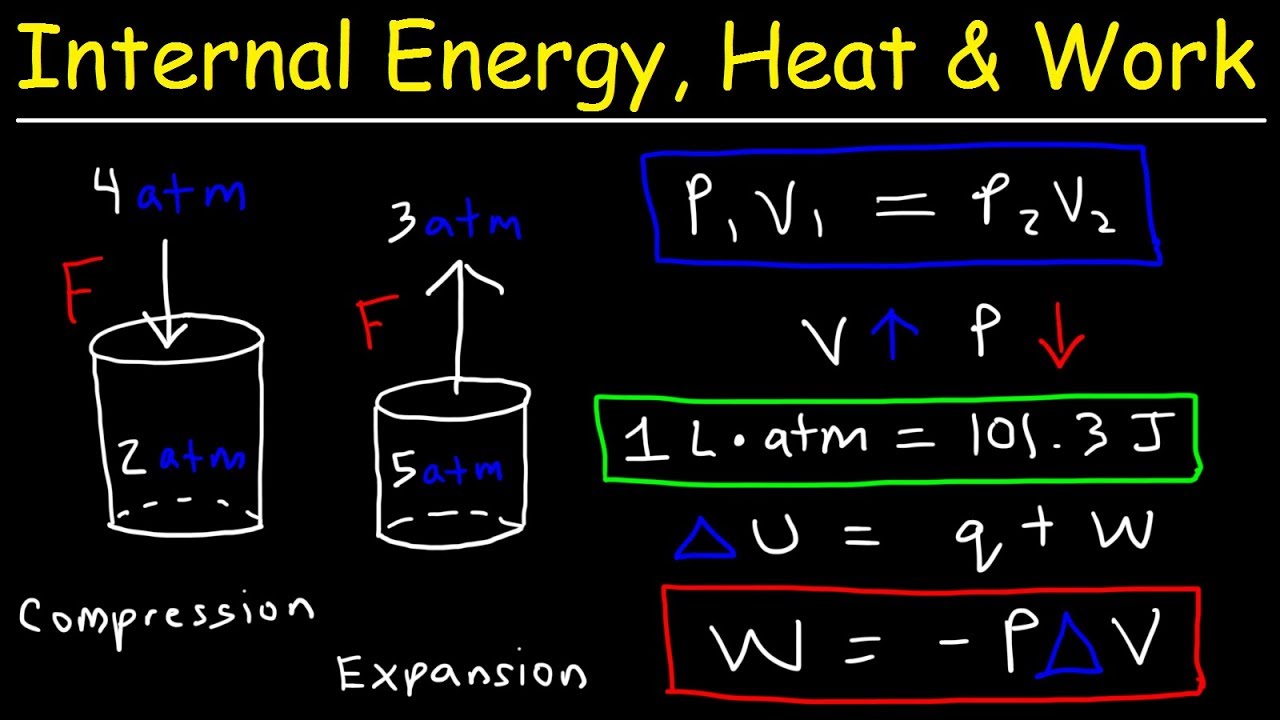
- 🔩 Work (w) is equal to negative pressure times change in volume (pΔV), and is positive when work is done on the system, negative when done by the system.
- 📐 One liter-atmosphere (1 L·atm) is equivalent to 101.3 joules, which is a conversion factor for work done during gas expansion or compression.
- ⚖️ The mass (m), specific heat capacity (c), and change in temperature (ΔT) are used in the equation q = mcΔT to calculate heat energy for temperature changes.
- 🧊 The heat of fusion for water is about six kilojoules per mole, which is needed to convert ice to liquid water at constant temperature.
- 🔥 The combustion of propane is an exothermic reaction releasing 1200 kilojoules of heat energy, which can be calculated using the balanced chemical equation.
- 📉 The enthalpy of formation is the heat absorbed or released when a compound is produced from its elements in their natural standard state.
- ⚖️ Hess's Law allows the calculation of the enthalpy change for a reaction by manipulating and adding other known reactions.
- 🔍 The enthalpy change of a reaction can be estimated by subtracting the sum of the heats of formation of the reactants from the sum of the heats of formation of the products.
- 📐 To convert between grams and moles, use the molar mass from the periodic table, and to convert moles to grams for substances like CO2, use the molar mass which is 44 grams per mole.
Q & A
What is the formula representing the change in internal energy of a system?
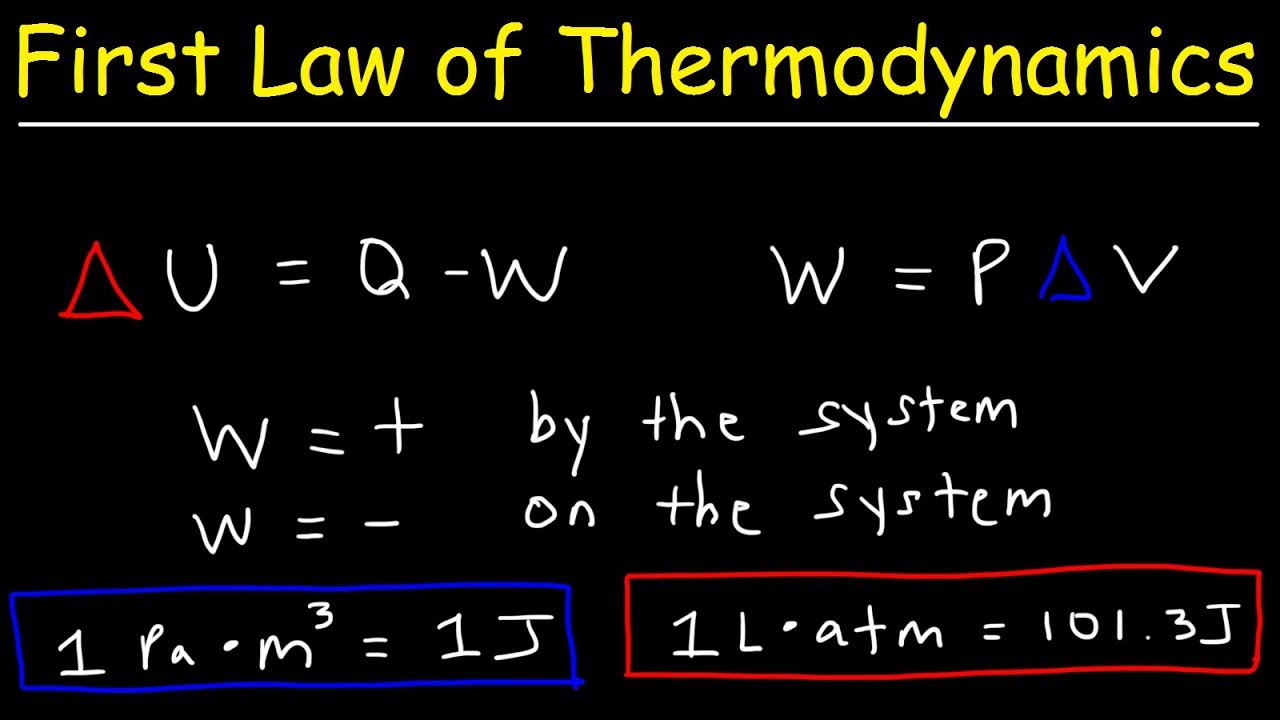
-The change in the internal energy of a system, denoted as ΔE, is equal to the heat energy added to the system (q) plus the work done on the system (w), which can be represented as ΔE = q + w.
How is heat flow related to the temperature difference between a system and its surroundings?
-Heat flows from a region of higher temperature to a region of lower temperature. If the system is at a higher temperature than the surroundings, heat will flow out of the system, making q negative with respect to the system.
What is the sign of q when heat is absorbed by the surroundings?
-When the surroundings absorb heat energy, q is positive, indicating an endothermic process for the surroundings.
How is work (w) defined in the context of a system and its surroundings?
-Work (w) is defined as the product of pressure (p) and change in volume (ΔV), and is given by the formula w = -pΔV. If work is done on the system, w is positive; if work is done by the system, w is negative.
What is the conversion factor between liters times atmosphere (atm) and joules?
-One liter times one atmosphere is equal to 101.3 joules.
How can you calculate the change in internal energy when a gas expands?
-To calculate the change in internal energy (ΔE) when a gas expands, you first calculate the work done using the formula w = -pΔV, then use the equation ΔE = q + w, where q is the heat energy absorbed or released.
What is the formula to calculate heat energy (q) during a temperature change?
-The formula to calculate heat energy (q) during a temperature change is q = mcΔT, where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
What is the heat of fusion for water in kilojoules per mole?
-The heat of fusion for water is about six kilojoules per mole.
How do you determine if a reaction is exothermic or endothermic based on the given heat energy?
-If the heat energy is given on the right side of the reaction, it is exothermic (releases heat). If it is on the left side, it is endothermic (absorbs heat).
How can you calculate the enthalpy change for a reaction using the heats of formation?
-You can calculate the enthalpy change for a reaction using the formula ΔH = ΣΔH(products) - ΣΔH(reactants), where ΔH is the heat of formation for each compound.
What is Hess's Law and how is it used to estimate the enthalpy change of a reaction?
-Hess's Law states that the total enthalpy change for a chemical reaction is the same, no matter how the reaction is carried out, in a single step or in a series of steps. It is used to estimate the enthalpy change of a reaction by manipulating and adding two or more known reactions to produce the target reaction.
How do you convert grams of a substance to moles using the molar mass?
-To convert grams to moles, you divide the mass of the substance in grams by its molar mass. The molar mass can be found on the periodic table and is the mass of one mole of that substance.
Outlines
🔥 Internal Energy and Heat Transfer
This paragraph introduces the concept of internal energy and the equation ΔE = Q + W, where ΔE is the change in internal energy, Q is the heat energy, and W is the work done. It explains that Q is negative when heat leaves the system (exothermic process) and positive when heat enters the system (endothermic process). The paragraph also covers the measurement of heat in joules and the conversion factors between kilojoules, joules, and calories. Work is represented by W and is calculated using the formula W = -PΔV, where P is pressure, and ΔV is the change in volume. The sign of W depends on whether work is done on or by the system. Finally, it presents a practice problem involving the absorption of heat by a gas and the expansion of the gas to calculate the change in internal energy.
🌡️ Calculation of Heat Energy with Specific Heat Capacity
The second paragraph delves into the calculation of heat energy using the formula Q = mcΔT, where m is mass, c is specific heat capacity, and ΔT is the change in temperature. It uses water as an example, noting its specific heat capacity is 4.184 joules per gram per degree Celsius. An example calculation is provided for heating 50 grams of water from 25°C to 75°C, resulting in the requirement of 10,460 joules of energy. The paragraph also explains how to calculate heat energy during phase changes using the formula Q = mΔH or Q = nΔH, depending on the units of enthalpy change (ΔH). It further illustrates the calculation using the heat of fusion for water as an example, converting grams of ice to moles and then calculating the energy required to melt the ice into water.
🔍 Balancing Chemical Equations and Calculating Heat Energy
This paragraph discusses thermal chemical equations, using the combustion of propane as an example. It outlines how to balance chemical equations by ensuring the number of atoms for each element is the same on both sides. The paragraph also explains how to calculate the heat energy released during a reaction using the molar ratio, converting grams to moles and then to kilojoules. An example is given where 64 grams of oxygen reacts, and the resulting kilojoules of heat energy released are calculated. The concept of endothermic and exothermic reactions is also explained, noting the placement of heat energy values on either side of the reaction arrow.
📉 Enthalpy of Reaction and Formation
The fourth paragraph focuses on the enthalpy of formation and how it can be used to estimate the enthalpy of a reaction. Enthalpy of formation is defined as the heat absorbed or released when a compound is produced from its elements in their natural standard state. The paragraph provides an example using methane and oxygen to form carbon dioxide and water, with given heats of formation for CO2, H2O, and CH4. It demonstrates how to calculate the enthalpy of the reaction by summing the products' enthalpies and subtracting the reactants' enthalpies. The calculation results in an estimated enthalpy of -184 kilojoules per mole for the reaction.
🔧 Hess's Law and Reaction Enthalpy Calculation
The final paragraph introduces Hess's Law, which is used to estimate the enthalpy of a reaction by manipulating and adding two known reactions to match a third, unknown reaction. An example is given with three reactions, where the enthalpies for the first and second reactions are provided. By doubling the first reaction and reversing the second, the paragraph shows how to achieve the third reaction and add the enthalpies to find the enthalpy for the net reaction, which is calculated to be 800 kilojoules in the example. This method is a practical application of Hess's Law for estimating reaction enthalpies.
Mindmap
Keywords
💡Internal Energy
💡Heat (q)
💡Work (w)
💡Energy Conversion
💡Thermal Chemistry
💡Specific Heat Capacity
💡Phase Change
💡Enthalpy of Fusion
💡Thermal Chemical Equation
💡Enthalpy of Formation
💡Hess's Law
Highlights
The change in internal energy of a system is equal to the sum of heat (q) and work (w).
Heat (q) is negative when it flows out of the system and positive when absorbed by the surroundings.
Work (w) is positive when done on the system and negative when done by the system, calculated as w = -pΔV.
1 kilojoule equals 1000 joules, and 1 calorie equals 4.184 joules.
The internal energy of a system increases when work is done on it and decreases when the system does work.
101.3 joules is equivalent to one liter times one atmosphere of pressure.
An example calculation of the internal energy change when 300 joules of heat is absorbed and the gas expands from 2 liters to 3 liters at 5 atm.
q can be calculated using the equation mCΔT, where m is mass, C is specific heat capacity, and ΔT is the change in temperature.
For phase changes, q can be calculated using q = mΔH, where ΔH is the enthalpy of fusion or vaporization.
The heat of fusion for water is about 6 kilojoules per mole.
Thermal chemical equations show the amount of heat energy released or absorbed without a ΔH symbol.
Balancing combustion reactions involves balancing carbon and hydrogen atoms first, then oxygen.
Enthalpy of formation (ΔHf) is the heat absorbed or released when a compound is formed from its elements in their natural standard state.
Hess's Law allows the estimation of the enthalpy change of a reaction by manipulating and adding other known reactions.
Enthalpy of a reaction can be estimated using the sum of products' enthalpies minus the sum of reactants' enthalpies.
Example calculation of the enthalpy change for a methane combustion reaction using heats of formation.
Enthalpy change can be calculated for a reaction given two other reactions and their enthalpies using Hess's Law.
Transcripts
Browse More Related Video
Thermochemistry Equations and Formulas With Practice Problems
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

AP Chem Unit 6 Review - Thermodynamics in 10 Minutes!
First Law of Thermodynamics, Basic Introduction, Physics Problems

Enthalpy | Thermodynamics

Work from expansion | Thermodynamics | Physics | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: