Work from expansion | Thermodynamics | Physics | Khan Academy
TLDRThis script discusses how internal energy in a system can change through heat transfer or work done by or on the system. It explains heat transfer through temperature differences and work through pressure and volume changes, using a piston example to illustrate how work is calculated as pressure times change in volume.
Takeaways
- 🔥 The change in internal energy of a system can be due to heat transfer or work done on/by the system.
- 🌡️ Heat transfer occurs when a system at a lower temperature comes into contact with a system at a higher temperature, causing energy to flow from the hotter to the cooler system.
- 🔨 Work can be done by a system when it exerts pressure to move a boundary, such as a piston, against an opposing force.
- 💥 The concept of heat and work is related to the macrostates of a system, such as temperature and pressure, but also involves microstates like molecular kinetic energy.
- 🧱 Heat transfer involves the kinetic energy exchange at the molecular level, facilitated by collisions with container walls or other surfaces.
- 📐 The formula for work in the context of a piston and gas system is work = force x distance, where force is pressure x area, and distance is the change in volume.
- 🔧 Work done by a system can be expressed as the product of pressure and the change in volume (∆V), which is a key concept in understanding engines and other mechanical systems.
- 🔄 The internal energy change of a system can be rewritten as the heat added to the system minus the work done by the system, which is pressure times volume change.
- 🚗 This principle of work and energy is fundamental to the operation of engines, where an internal explosion pushes a piston, which in turn moves other components.
- 📉 When the volume of a system increases, the system is doing work, which is a crucial aspect of energy conversion in various mechanical processes.
- 📈 The script suggests that future discussions will relate these concepts to the PV (pressure-volume) diagram, indicating a deeper exploration of thermodynamic processes.
Q & A
How can the internal energy of a system change?
-The internal energy of a system can change due to the addition or removal of heat, or through work being done on or by the system.
What is the relationship between heat and the macrostate of a system?
-Heat is responsible for changing the macrostate of a system, such as its temperature, by transferring kinetic energy between particles.
How does heat transfer occur between two systems?
-Heat transfer occurs from a system at a higher temperature to one at a lower temperature, typically through conduction via a medium or direct contact.
What happens at the microscopic level during heat transfer?
-At the microscopic level, molecules in the hotter system lose kinetic energy, while those in the cooler system gain kinetic energy, facilitated by collisions and vibrations.
How can work be added or subtracted from a system?
-Work can be added or subtracted from a system through processes like compression or expansion, where external forces are applied to the system.
What is a quasi-static process in thermodynamics?
-A quasi-static process is one that is slow enough to be considered close to equilibrium at all times, allowing for the use of macroscopic properties like pressure and volume.
How does the pressure of a gas affect the work done by a system?
-The pressure of a gas can do work on a system by pushing against a piston, which can be calculated as the product of pressure, area, and the distance the piston moves.
What is the formula for calculating work done by a system in terms of pressure and volume?
-The work done by a system can be calculated as the pressure of the system times the change in volume (∆V).
How is the internal energy change of a system related to heat and work?
-The change in internal energy of a system is equal to the heat added to the system minus the work done by the system (∆U = Q - W).
What is the significance of the relationship between work and volume change in engines?
-In engines, the expansion of gases (increase in volume) does work by pushing a piston, which is a fundamental principle in converting thermal energy into mechanical work.
Outlines
🔥 Understanding Heat Transfer and Work
This paragraph discusses the concepts of heat transfer and work in relation to a system's internal energy. It explains that the change in internal energy can be due to heat being added or work being done on the system. The speaker uses the example of a system at temperature T1 (300 Kelvin) and another at a higher temperature T2 (1000 Kelvin) to illustrate how heat is transferred from the hotter system to the cooler one. The paragraph also touches on the idea that heat changes the macrostate of a system, affecting the kinetic energy of its molecules. The concept of work is introduced through the example of a piston system, where work is done when the pressure of the gas inside the container pushes the piston up, causing a change in the system's volume.
🔧 Work Done by a System: The Piston Example
This paragraph delves deeper into the concept of work, particularly how it is done by a system. The speaker uses the example of a piston being pushed up by the pressure of a gas inside a container. The work done by the system is equated to the force applied to the piston, which is the product of pressure and the area of the piston. The distance the piston moves (x) is then used to calculate the work done, which is the product of force and distance. The speaker further explains that this force is equivalent to the pressure times the area of the piston, and the distance moved is part of the change in volume of the container. This leads to the conclusion that work done by the system can be expressed as the pressure times the change in volume.
🚀 Relating Internal Energy to Heat and Work
In this paragraph, the speaker connects the concepts of internal energy, heat, and work. The internal energy change of a system is described as the heat added to the system minus the work done by the system. The work done by the system, as previously explained, is the pressure times the change in volume. This relationship is then used to rewrite the formula for internal energy change, emphasizing the role of work in the process. The speaker also hints at the relevance of this concept in the context of engines, where the expansion of gas in a cylinder does work by pushing a piston, which in turn moves other components to generate motion. The paragraph concludes by setting the stage for future discussions on how these concepts relate to the PV (pressure-volume) diagram.
Mindmap
Keywords
💡Internal Energy
💡Heat
💡Work
💡Temperature
💡Kinetic Energy
💡Pressure
💡Volume
💡Quasi-static Process
💡Macrostates
💡Microstates
💡PV Diagram
Highlights
The internal energy of a system can change due to heat transfer or work done on/by the system.
Heat is transferred from a higher temperature system to a lower temperature system.
Heat transfer involves kinetic energy exchange at the molecular level through collisions.
Work can be done on a system by applying force over a distance, as in the case of a piston being pushed up.
Pressure is defined as force per unit area and is a key factor in work done by a system.
The work done by a system can be expressed as pressure times the change in volume.
Quasi-static processes are those close to equilibrium, allowing the discussion of macrostates like pressure and volume.
Macrostates like temperature, pressure, and volume change as a result of heat and work interactions.
The concept of macrostates and microstates is crucial for understanding heat and work at a fundamental level.
Heat transfer can intuitively be understood through the example of particles in a canister at different temperatures.
The work done on a system can be visualized using a piston and pebbles model to represent pressure.
The formula for work done by a system is derived from the basic principles of force, pressure, area, and distance.
The internal energy change formula can be rewritten incorporating the work done by the system in terms of pressure and volume change.
The relationship between heat, work, and internal energy is fundamental to understanding thermodynamic processes.
Practical applications of these concepts are evident in engines, where work is done by expanding gases.
The video promises to further explore the connection between the internal energy formula and the PV diagram in the next installment.
Transcripts
Browse More Related Video
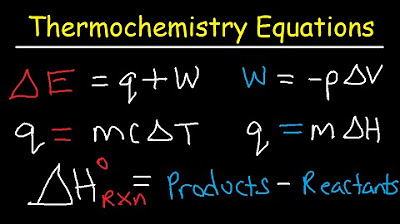
Thermochemistry Equations & Formulas - Lecture Review & Practice Problems

AP Physics B - 2013 #5 (Thermodynamics - Work)

Work done by isothermic process | Thermodynamics | Physics | Khan Academy
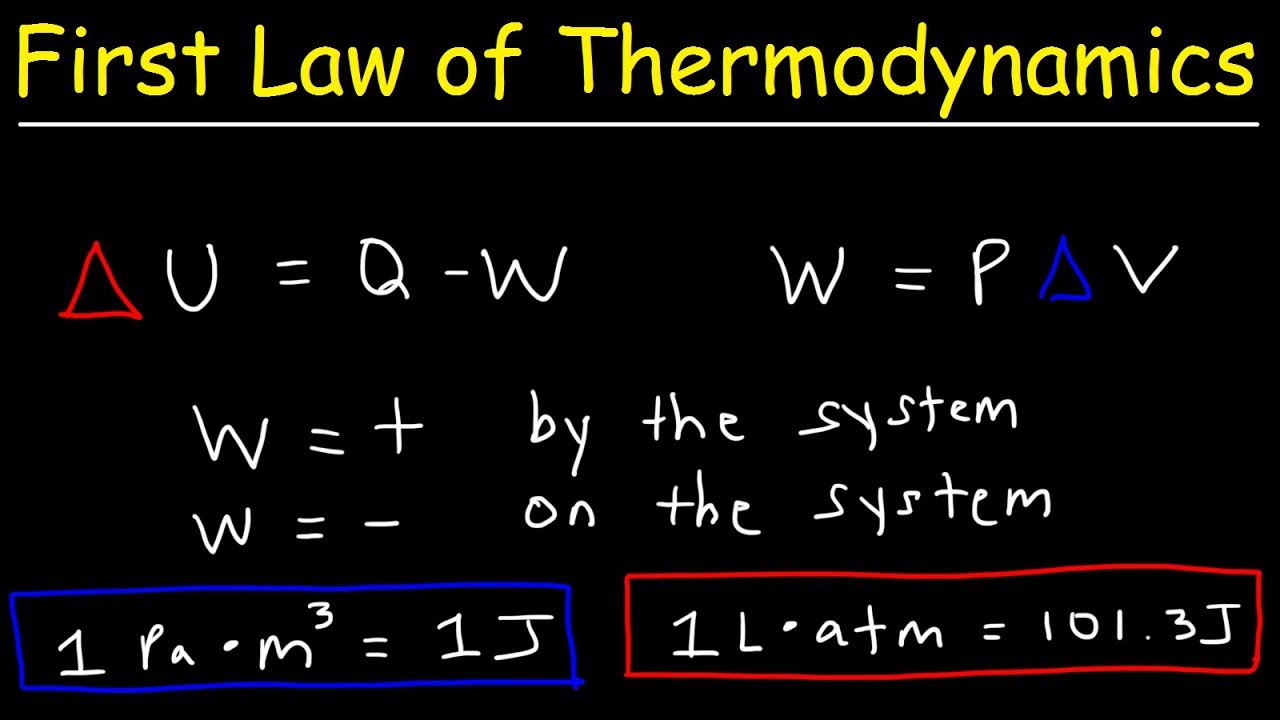
First Law of Thermodynamics, Basic Introduction, Physics Problems
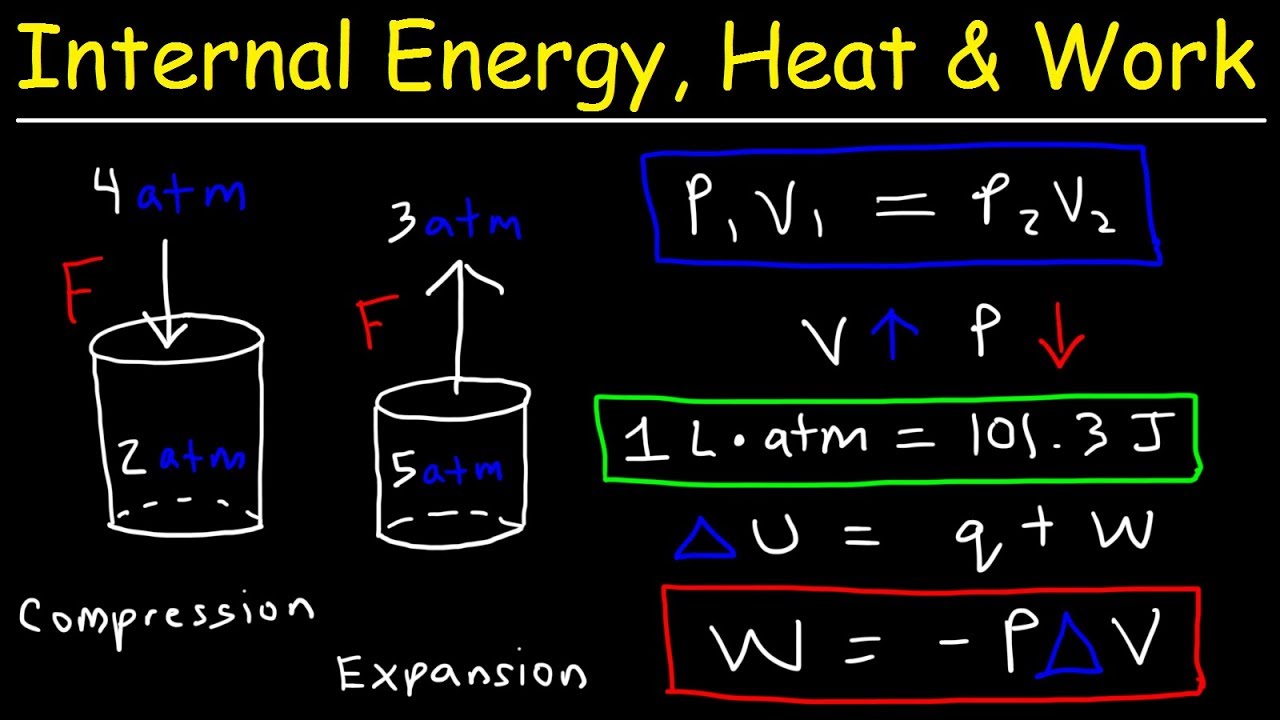
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

PV-diagrams and expansion work | Thermodynamics | Physics | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: