Thermochemistry Equations and Formulas With Practice Problems
TLDRThis educational video delves into thermal chemistry with practice problems. It explains how to calculate work done by a gas expanding against pressure, using the formula W = -PΔV, and demonstrates the concept with a step-by-step example. The video also covers calculating heat absorbed during temperature and phase changes, employing specific heat capacity and heat of fusion. Finally, it teaches how to determine the enthalpy change of reactions, including combustion, using heats of formation and thermochemical equations, providing a comprehensive guide to understanding and applying these principles.
Takeaways
- 🔥 The video covers practice problems in thermal chemistry, focusing on concepts like work done by a system, internal energy, heat absorption, and enthalpy changes.
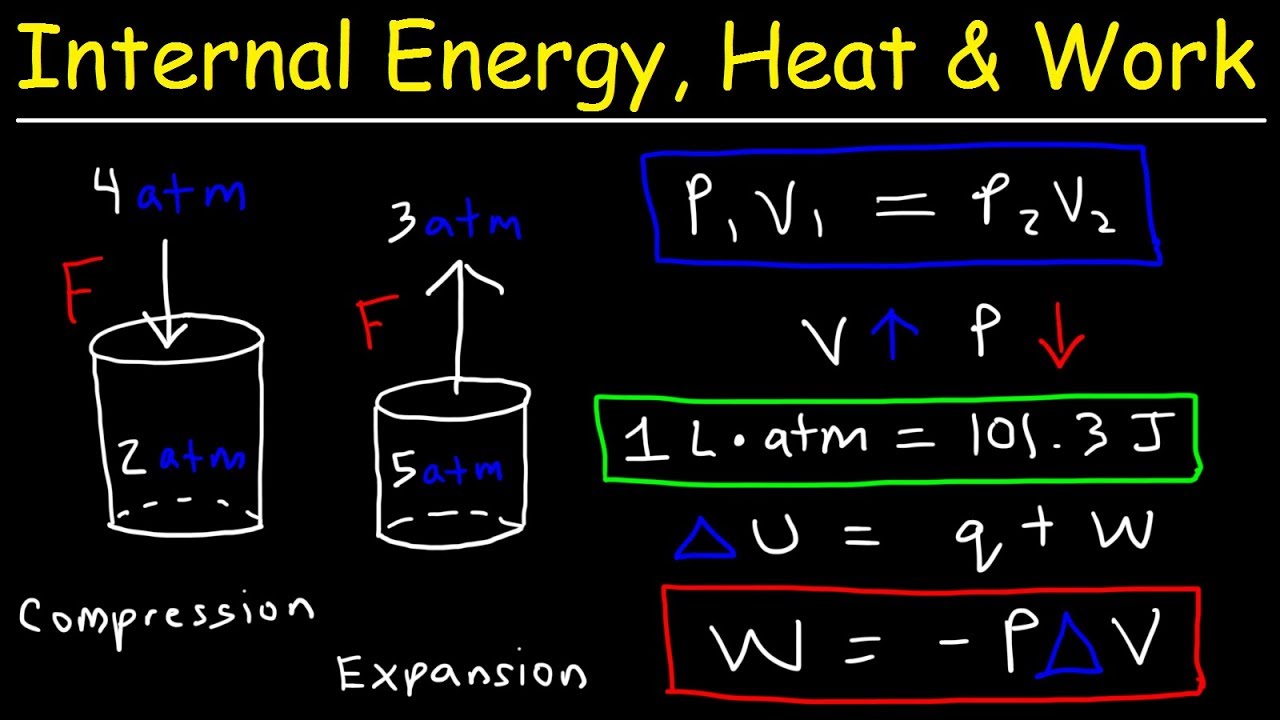
- 🌡️ It explains that when a gas absorbs heat (endothermic process), it expands and does work on its surroundings, which can be calculated using the formula W = -PΔV where W is work, P is pressure, and ΔV is change in volume.
- 📚 The script clarifies the difference between work done by the system (negative W) and work done on the system (positive W) in the context of gas expansion and compression.
- ⚗️ It demonstrates the calculation of internal energy change (ΔE) using the formula ΔE = Q + W in chemistry, and the distinction from the physics convention.
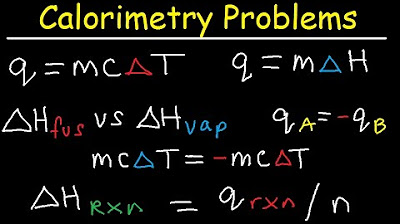
- 💧 The video provides a method to calculate the heat energy absorbed by water when its temperature changes, using the formula Q = mcΔT where m is mass, c is specific heat capacity, and ΔT is the temperature change.
- 🧊 The script explains how to calculate the heat energy released during the phase change, such as the freezing of water to ice, using the heat of fusion and molar conversions.
- 🔥 The concept of enthalpy of combustion is introduced, which quantifies the amount of heat energy released when a substance reacts completely with oxygen.
- 📉 The video illustrates how to calculate the enthalpy change for a reaction using the standard heats of formation, emphasizing the difference between products and reactants.
- 📚 It emphasizes the importance of understanding the direction of physical changes (solid to liquid, liquid to gas) to determine whether energy is released or absorbed.
- 🔢 The script uses specific examples, such as the combustion of propane and methane, to demonstrate the calculation of heat energy released during reactions.
- 🌡️ The importance of specific heat capacity in determining a substance's ability to store thermal energy is highlighted, with examples comparing different substances.
Q & A
What is the process described in the video for calculating the work done by a gas expanding at constant pressure?
-The process involves using the formula W = -PΔV, where W is the work done by the system, P is the constant pressure, and ΔV is the change in volume. The work is negative when the system does work by expanding.
Why is the heat absorbed by the system considered positive in the script?
-In the script, the heat absorbed by the system is considered positive because it represents an endothermic process where thermal energy flows from the surroundings into the system.
How does the video explain the relationship between the change in volume (ΔV) and the work done by the system?
-The video explains that when ΔV is positive, indicating gas expansion, the work done by the system (W) is negative. Conversely, when ΔV is negative, indicating compression, W is positive as work is done on the system.
What is the significance of the conversion factor 1 liter-atm to joules in the script?
-The conversion factor is significant because it allows the calculation of work done in terms of energy in joules. It is used to convert the product of liters and atmospheres into joules, which is necessary for calculating the work done by the gas.
How is the internal energy change of the system calculated in the script?
-The internal energy change (ΔE) is calculated using the formula ΔE = Q + W, where Q is the heat absorbed by the system and W is the work done by the system. The video emphasizes that in chemistry, W is considered negative when work is done by the system.
What is the difference between the specific heat capacity of water and a hypothetical substance A with a specific heat capacity of 0.1 J/g°C?
-The specific heat capacity of water (4.184 J/g°C) is much higher than that of substance A (0.1 J/g°C), meaning water can absorb more heat per degree Celsius per gram without a significant change in temperature compared to substance A.
How does the video calculate the heat energy absorbed when 70 grams of water's temperature rises from 25°C to 60°C?
-The calculation uses the formula Q = mCΔT, where m is the mass of water (70 grams), C is the specific heat capacity of water (4.184 J/g°C), and ΔT is the change in temperature (35°C). The result is then converted to kilojoules.
What is the concept of heat of fusion, and how is it used in the script to calculate the heat energy released when water freezes?
-The heat of fusion is the amount of energy released when a substance changes from liquid to solid state. In the script, it is used with the formula Q = nΔHf, where n is the number of moles of water and ΔHf is the heat of fusion per mole, to find the total energy released during freezing.
How does the video approach the calculation of the heat energy released during the combustion of 88 grams of propane?
-The video uses the balanced chemical equation for the combustion of propane and the enthalpy of combustion per mole to find the heat energy released. It first converts the mass of propane to moles and then multiplies by the enthalpy of combustion to get the total energy released.
What is the standard heat of formation, and how is it used in the script to calculate the standard enthalpy of combustion for methane?
-The standard heat of formation is the change in enthalpy during the formation of one mole of a substance from its constituent elements in their standard states. In the script, it is used in the formula for calculating the enthalpy of reaction, which for a combustion reaction is equivalent to the enthalpy of combustion.
Outlines
🔥 Thermal Chemistry Practice Problems
The video begins with an introduction to practice problems in thermal chemistry. It covers a scenario where a gas absorbs 2500 joules of heat while expanding from 3 liters to 7 liters at a constant pressure of 4 atm. The process is explained as endothermic, with the surroundings losing thermal energy. The concept of work done by the system during gas expansion is introduced, using the formula W = -PΔV, where W is work, P is pressure, and ΔV is the change in volume. The video emphasizes the sign convention for work done by or on the system and illustrates it with an example involving two cylinders.
📚 Calculating Work and Internal Energy in Chemistry
This paragraph delves into the calculation of work done by a system during gas expansion, using the formula W = -PΔV, and explains the importance of the sign of ΔV in determining whether work is done by or on the system. It then transitions to calculating the change in internal energy (ΔE) of the system, using the relationship ΔE = Q + W, where Q is the heat absorbed and W is the work done. The difference between the conventions used in chemistry and physics for these calculations is highlighted, and an example calculation is provided to show how the internal energy of the system changes when it absorbs heat and does work.
💧 Heat Absorption by Water During Temperature Change
The script moves on to calculate the heat energy absorbed by 70 grams of water as its temperature rises from 25°C to 60°C. The formula Q = mCΔT is introduced, where m is mass, C is the specific heat capacity of water, and ΔT is the change in temperature. The calculation results in 10250 joules, which is then converted to kilojoules, illustrating the high specific heat capacity of water and its ability to store thermal energy.
🧊 Understanding Specific Heat Capacity and Phase Change
This section discusses the concept of specific heat capacity and its relation to a substance's ability to store thermal energy. It provides an example comparing two substances with different specific heat capacities and how they respond to the absorption of one joule of heat energy. The explanation highlights the difference between substances that are good at storing heat energy, like water, and those that are good at transferring heat energy, like metals.
❄️ Heat Energy Released During Freezing of Water
The video script addresses the calculation of heat energy released when 72 grams of liquid water freezes into ice at 0°C. It recommends a conversion process using the molar mass of water and the heat of fusion, which is the energy released when one mole of water freezes. The calculation involves converting grams to moles and then using the heat of fusion to find the energy released, resulting in -24 kilojoules, indicating an exothermic process.
🔥 Enthalpy of Combustion of Propane
The script explains how to calculate the heat energy released during the complete combustion of 88 grams of propane with oxygen gas. It involves writing the balanced chemical equation for the combustion of propane and using the enthalpy of combustion, which is the amount of heat energy released when one mole of a substance combusts. The calculation converts the mass of propane to moles and then applies the enthalpy of combustion to find the total energy released, resulting in -4440 kilojoules.
🌡️ Calculating Standard Enthalpy of Combustion for Methane
The final part of the script focuses on calculating the standard enthalpy of combustion for methane using the standard heats of formation for methane, CO2, and H2O. It involves writing the balanced combustion reaction for methane and applying Hess's law to calculate the enthalpy change of the reaction. The calculation uses the given standard heats of formation and results in a negative value, indicating an exothermic reaction and the amount of heat energy released during the combustion of one mole of methane.
Mindmap
Keywords
💡Thermal Chemistry
💡Joules
💡Endothermic Process
💡Work Done by the System
💡Constant Pressure
💡Internal Energy
💡Specific Heat Capacity
💡Heat of Fusion
💡Enthalpy of Combustion
💡Standard Heat of Formation
Highlights
The video covers practice problems in thermal chemistry, starting with a problem involving a gas absorbing heat and expanding at constant pressure.
A visual explanation is provided for how heat energy flows from surroundings to the system, and the concept of endothermic and exothermic processes.
The formula for calculating work done by a system during gas expansion is introduced, emphasizing the relationship between volume change and work.
The convention of work done by the system being negative during expansion is explained, with a visual aid to clarify the concept.
A step-by-step calculation of work done by the gas during expansion, using the given heat energy, volume change, and constant pressure, is demonstrated.
The conversion of units from liters times atm to joules is discussed, with a specific conversion factor provided.
The calculation of the internal energy change of the system is explained, highlighting the difference in sign conventions between chemistry and physics.
An analogy is used to compare the internal energy change to a bank account, illustrating the concept of energy flowing into and out of the system.
The calculation of heat energy absorbed by water during a temperature rise is shown, using the specific heat capacity formula.
The concept of specific heat capacity is explained, with an example comparing two substances with different capacities to store thermal energy.
The heat energy released during the freezing of water into ice is calculated, using the heat of fusion and molar mass.
The direction of physical changes and the sign of energy release or absorption in phase changes are discussed.
The enthalpy of combustion of propane is used to calculate the heat energy released during its complete reaction with oxygen.
A balanced chemical equation for the combustion of propane is presented, with a focus on the stoichiometry of the reaction.
The concept of thermochemical equations linking the amount of thermal energy with a chemical reaction is introduced.
The standard heat of formation values for methane, CO2, and water are used to calculate the standard enthalpy of combustion for methane.
A formula for calculating the enthalpy of reaction using heats of formation is provided, with an example calculation for methane combustion.
The video concludes with a summary of the key concepts covered, including work done by a gas, internal energy change, heat absorption during temperature and phase changes, and enthalpy change of reactions.
Transcripts
Browse More Related Video
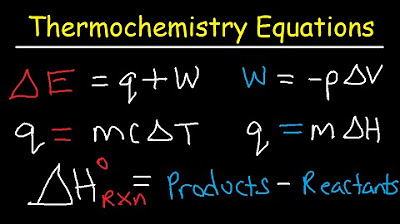
Thermochemistry Equations & Formulas - Lecture Review & Practice Problems
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems
Calorimetry Problems, Thermochemistry Practice, Specific Heat Capacity, Enthalpy Fusion, Chemistry

Calculating Enthalpy and Entropy Using the NIST WebBook

AP Chem Unit 6 Review - Thermodynamics in 10 Minutes!

Enthalpy Change of Reaction & Formation - Thermochemistry & Calorimetry Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: