Writing Chemical Formulas For Ionic Compounds
TLDRThis educational video script focuses on the principles of writing chemical formulas for ionic compounds. It emphasizes the importance of understanding the charges of ions, particularly for elements in groups one, two, and thirteen to fifteen of the periodic table. The script explains how elements like lithium, sodium, and potassium form +1 cations, while alkaline earth metals like calcium and magnesium form +2 cations. Transition metals have variable charges and are not the focus of the lesson. Aluminum forms a +3 cation, while elements like nitrogen and phosphorus form -3 anions, and halogens form -1 anions. The video teaches how to balance charges by using a one-to-one ratio for ions with equal and opposite charges, and the crisscross method for those with different magnitudes. It also covers how to handle polyatomic ions and provides an example with barium phosphate. Finally, it touches on transition metals, using iron sulfate as an example, and concludes with a reminder of the significance of charge balance in forming neutral compounds.
Takeaways
- 🔋 To write chemical formulas for ionic compounds, you need to know the charges of ions.
- 🌟 Group 1 elements (like lithium, sodium, potassium) form +1 charge cations.
- 🌕 Group 2 elements (alkaline earth metals like calcium, magnesium) form +2 charge ions.
- 📊 Transition elements can have variable charges and are not the focus of this lesson.
- 📍 Group 13 elements (like aluminum) form +3 charge cations.
- ⚙️ Group 4a elements (like carbon, silicon, germanium) are rarely seen in ionic compounds.
- 🟣 Group 5a elements (like nitrogen, phosphorus) form -3 charge anions.
- 🟡 Group 6a elements (like oxygen, sulfur, selenium) form -2 charge anions.
- 🟢 Halogens (like fluoride, chloride, bromide, iodide) form -1 charge anions.
- ⚖️ When writing formulas, ions with equal and opposite charges combine in a one-to-one ratio.
- 🔀 If charges differ, use the crisscross method to swap charges with subscripts to balance the formula.
- 📐 For polyatomic ions, enclose the ion in parentheses and use the crisscross method to determine the formula.
- 📚 Roman numerals indicate the charge on metals, especially in transition metal compounds like iron(III) sulfate.
Q & A
What is the charge of ions formed by elements in Group 1 of the periodic table?
-Elements in Group 1, such as lithium, sodium, and potassium, form cations with a positive one charge.
Which group of elements are known as alkaline earth metals and what charge do their ions typically have?
-Group 2 elements are known as alkaline earth metals, which include calcium and magnesium, and their ions typically have a two plus charge.
What is the charge of aluminum ions?
-Aluminum, which is found in Group 13 or 3a, forms cations with a three plus charge.
What is the charge of ions formed by elements in Group 5a?
-Elements in Group 5a, such as nitrogen and phosphorus, form anions with a negative three charge.
How do halogens typically form ions and what is the charge of these ions?
-Halogens, which include elements like fluoride, chloride, bromide, and iodide, form anions with a negative one charge.
How is the chemical formula for sodium bromide written?
-The chemical formula for sodium bromide is written as NaBr, since both sodium and bromide ions have charges of equal magnitude but opposite signs, combining in a one-to-one ratio.
What is the process of writing the chemical formula for calcium sulfide?
-The chemical formula for calcium sulfide is written as CaS. Calcium has a two plus charge and sulfide has a two minus charge, so they combine in a one-to-one ratio to neutralize each other.
How do you write the chemical formula for aluminum chloride when the charges are not equal?
-For aluminum chloride, with aluminum having a three plus charge and chloride a one minus charge, you use the crisscross method. Write the charge of aluminum as a subscript to the right of chlorine and omit the one, resulting in the formula AlCl3.
What is the chemical formula for sodium oxide and how do you determine it?
-The chemical formula for sodium oxide is Na2O. Sodium has a one plus charge and oxide (oxygen) has a two minus charge. Two sodium ions are needed to balance the charge of one oxide ion, hence the subscript '2' next to sodium.
How do you handle polyatomic ions when writing the chemical formula for an ionic compound?
-Polyatomic ions should be enclosed in parentheses and the total charge of the metal is crisscrossed with the subscript to the right of the polyatomic ion. For example, in barium phosphate, it is written as Ba3(PO4)2.
What does the Roman numeral next to a transition metal indicate in the chemical formula?
-The Roman numeral next to a transition metal indicates the charge on the metal ion. For example, in Iron(III) sulfate, the 'III' indicates that iron has a three plus charge.
How is the chemical formula for Iron(III) sulfate written and what is the significance of the Roman numeral?
-The chemical formula for Iron(III) sulfate is written as Fe2(SO4)3. The Roman numeral 'III' signifies that iron has a three plus charge, and sulfate (SO4) has a two minus charge, requiring two sulfate ions to balance the charge of one iron ion.
Outlines
🔬 Understanding Ionic Compounds and Charges
This paragraph introduces the concept of writing chemical formulas for ionic compounds. It emphasizes the importance of knowing the charges of ions, particularly for elements in groups one and two, as well as transition elements and those in groups 13 to 5a. The paragraph explains that group one elements like lithium, sodium, and potassium form +1 cations, group two elements like calcium and magnesium form +2 cations, while transition elements have variable charges. Group 13 elements like aluminum form +3 cations, and elements in group 5a like nitrogen and phosphorus form -3 anions. Oxygen, sulfur, and selenium form -2 anions, and halogens form -1 anions. The paragraph provides examples of how to write formulas for sodium bromide and calcium sulfide, explaining the one-to-one ratio when charges are equal in magnitude.
🧪 Writing Formulas with Unequal Charges
This paragraph discusses how to write chemical formulas when the charges of ions are not equal. It introduces a technique to balance the charges by swapping them with subscripts. For instance, aluminum chloride is formed by铝铝 (aluminum with a +3 charge) and chloride (a halogen with a -1 charge), resulting in the formula AlCl3. The paragraph also covers the writing of sodium oxide, where two sodium ions (Na+) are needed to balance one oxide ion (O2-), leading to the formula Na2O. It further explains the crisscross method for writing formulas with polyatomic ions, as demonstrated with barium phosphate, which is written as Ba3(PO4)2. The paragraph concludes with an example involving a transition metal, iron(III) sulfate, with the formula Fe2(SO4)3, highlighting the use of Roman numerals to denote the charge on the metal ion.
📝 Conclusion on Writing Ionic Compound Formulas
The final paragraph wraps up the lesson on writing chemical formulas for ionic compounds. It summarizes the method of balancing charges by using subscripts and emphasizes the importance of knowing the charges of ions for various elements. The paragraph thanks the viewers for watching and confirms that they now have the knowledge to write chemical formulas for ionic compounds.
Mindmap
Keywords
💡Ionic Compounds
💡Valence Electrons
💡Cations
💡Anions
💡Transition Elements
💡Polyatomic Ions
💡Charge Balancing
💡Subscripts
💡Chemical Formula Writing
💡Group Numbers
💡Crisscross Method
Highlights
The lesson focuses on writing chemical formulas for ionic compounds by understanding the charges of ions.
Elements in group one, like lithium, sodium, potassium, form cations with a +1 charge.
Group two elements, the alkaline earth metals, form ions with a +2 charge.
Transition elements can have variable charges, which are not the focus of this lesson.
Elements in group 13, like aluminum, form cations with a +3 charge.
Elements in group 5a, like nitrogen and phosphorus, form anions with a -3 charge.
Calcagen elements like oxygen, sulfur, selenium form anions with a -2 charge.
Halogens form anions with a -1 charge.
The charges of ions are crucial for determining the chemical formulas of ionic compounds.
Sodium bromide is formed by combining sodium (+1) and bromide (-1) ions in a 1:1 ratio.
Calcium sulfide is formed by combining calcium (+2) and sulfide (-2) ions in a 1:1 ratio.
Aluminum phosphide is formed by combining aluminum (+3) and phosphorus (-3) ions in a 1:1 ratio.
When charges are different, the crisscross method is used to balance the charges by swapping the charges with subscripts.
Aluminum chloride is formed by balancing the +3 charge of aluminum with three chloride (-1) ions.
Sodium oxide is formed by balancing the -2 charge of oxide with two sodium (+1) ions.
Barium phosphate is formed by balancing the +2 charge of barium with two phosphate (-3) ions, using parentheses for polyatomic ions.
Iron III sulfate is formed by balancing the +3 charge of iron with three sulfate (-2) ions.
The Roman numeral indicates the charge on the metal in ionic compounds.
Writing chemical formulas for ionic compounds involves neutralizing positive and negative charges to achieve a net charge of zero.
Transcripts
Browse More Related Video

What Are Ions | Properties of Matter | Chemistry | FuseSchool

Naming Ionic Compounds with Transition Metals Introduction

Writing Formulas with Polyatomic Ions

How to Predict Products of Chemical Reactions | How to Pass Chemistry
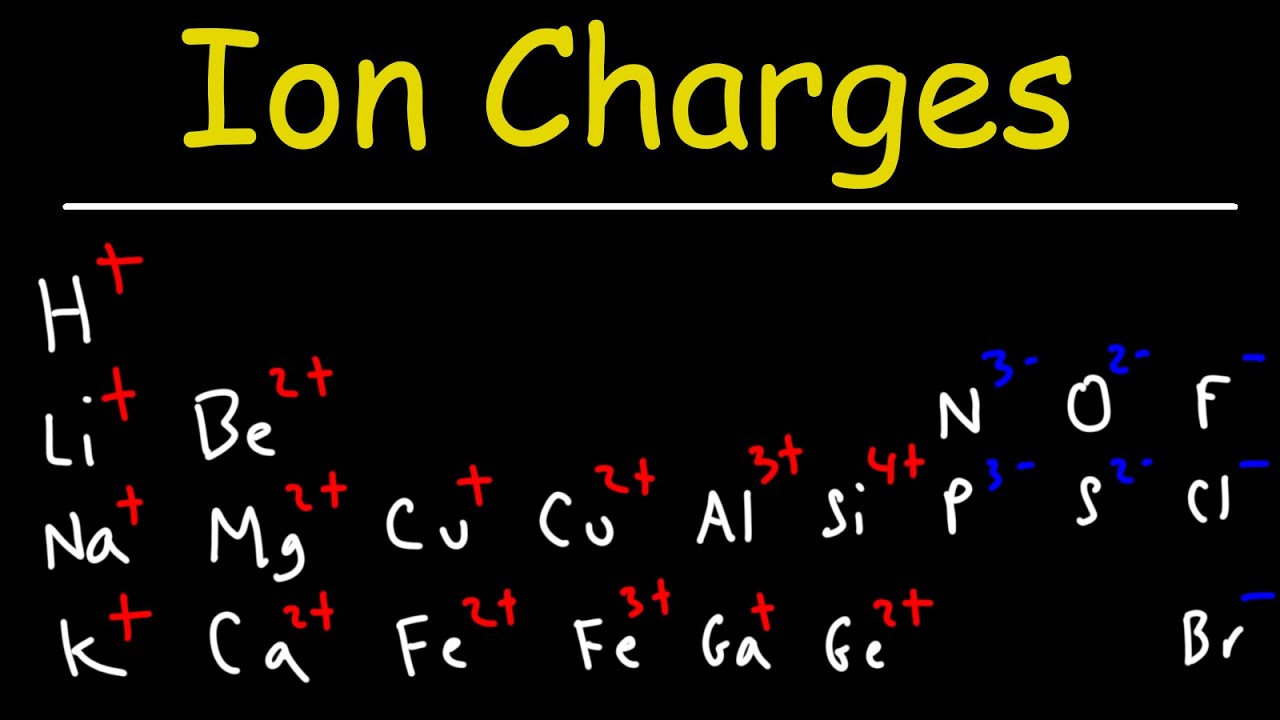
How To Determine The Charge of Elements and Ions - Chemistry

ALEKS: Deducing the ions in a polyatomic ionic compound from its empirical formula
5.0 / 5 (0 votes)
Thanks for rating: