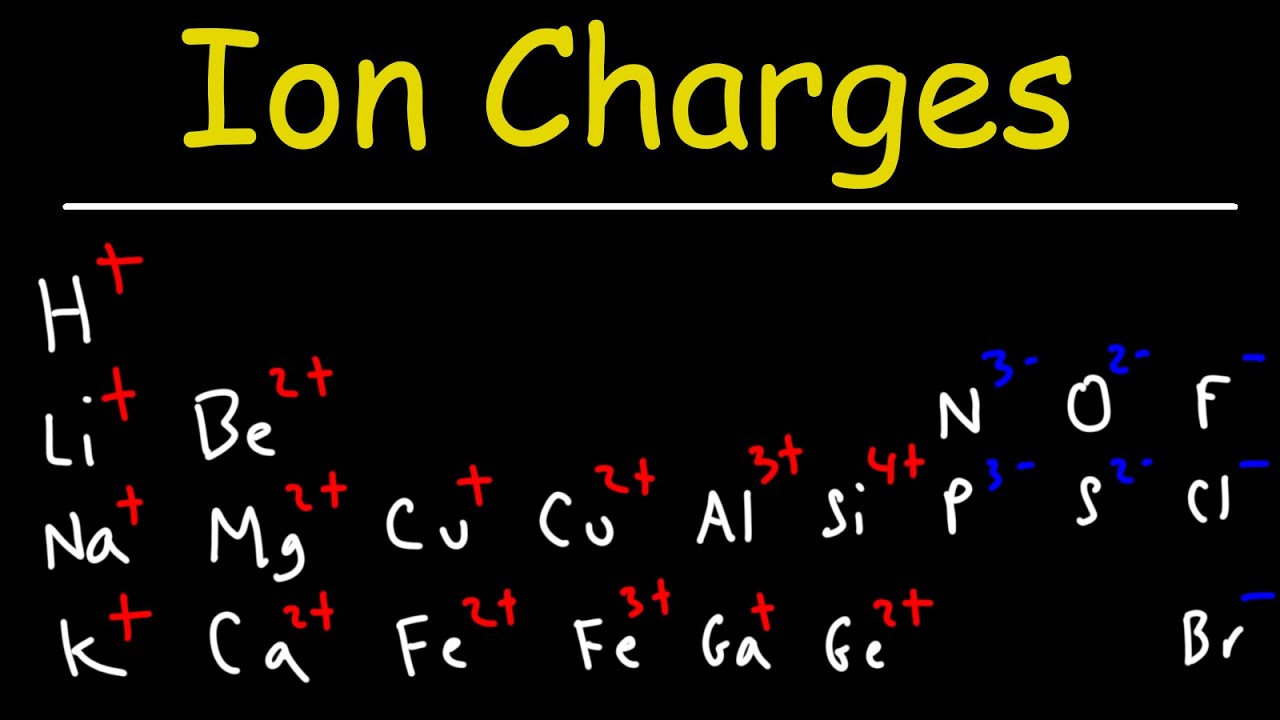What Are Ions | Properties of Matter | Chemistry | FuseSchool
TLDRThis lesson delves into the concept of ions, explaining how they are formed through the gain or loss of electrons by atoms, resulting in a charge. Positively charged ions, known as cations, are formed when an atom loses electrons, while negatively charged ions, called anions, are created when an atom gains electrons. The lesson uses sodium (Na) and oxygen (O) as examples, illustrating how these elements form ions with charges that result in a complete outer electron shell, mirroring the structure of noble gases. It also highlights common ion formation trends among metals and non-metals, such as Group 1 metals forming +1 cations and Group 6 non-metals like oxygen forming -2 anions. The summary emphasizes the importance of specifying charges when writing formulas and drawing electronic structures, providing a foundational understanding of ions and their role in chemistry.
Takeaways
- 🔬 An ion is an atom or molecule with a net electrical charge due to the loss or gain of one or more electrons.
- ⚡ When an atom loses electrons, it becomes positively charged and is known as a cation.
- ⚡ When an atom gains electrons, it becomes negatively charged and is known as an anion.
- 🐾 A mnemonic to remember cations: 'Cations' sounds like 'cats', which have 'paws', and 'paws' starts with a 'P', which stands for 'Positive'.
- 📊 The electronic structure of an atom is represented by a series of numbers, with the outermost shell being the valence shell.
- 📝 The charge of an ion is indicated as a superscript in its chemical formula and is included when drawing its electronic structure.
- 🧩 Ions have a complete outer shell, similar to the electronic structure of a noble gas, which is why they are stable.
- 🤔 For practice, draw the electronic structure of a calcium (Ca) and a chlorine (Cl) ion, noting their charges.
- 🤝 Metals, like Na and Mg, tend to lose valence electrons and form cations, with Group 1 metals forming +1 ions and Group 2 metals forming +2 ions.
- 💡 Non-metals, such as O and F, tend to gain electrons and form anions, with oxygen typically gaining two electrons to form O2- and fluorine gaining one electron to form F-.
- 📌 Group 3 elements form ions with a +3 charge, while Group 5 elements form ions with a -3 charge.
- 🔬 Group 4 elements, like carbon, often form covalent compounds rather than ionic ones.
- 📚 In summary, ions are charged particles formed by electron gain or loss, and they achieve stability by having the same electronic structure as the nearest noble gas.
Q & A
What is an ion?
-An ion is a charged particle that is formed when an atom gains or loses electrons.
Why do atoms develop a charge when they gain or lose electrons?
-Atoms develop a charge because electrons are negatively charged, so gaining electrons results in a negative charge, and losing electrons results in a positive charge.
What are positively charged ions called?
-Positively charged ions are called cations.
How can you remember the term for positively charged ions?
-You can remember it by associating 'cats' with 'paws', as both start with a 'c' and 'p' respectively, similar to 'cations' and 'positive'.
What are negatively charged ions called?
-Negatively charged ions are called anions.
What happens to the electronic structure of an atom when it forms an ion?
-When an atom forms an ion, it either loses or gains electrons to achieve a complete outer shell, resembling the electronic structure of a noble gas.
How is the charge of an ion represented in its formula?
-The charge of an ion is written as a superscript next to the element symbol in its formula.
What is the electronic structure of sodium (Na) and how does it form a cation?
-Sodium (Na) has an electronic structure of 2, 8, 1. It forms a cation by losing its one valence electron, resulting in a positively charged Na+ ion.
How does oxygen (O) form an anion and what is its charge?
-Oxygen (O) has an electronic structure of 2, 6 and forms an anion by gaining two electrons, achieving a complete octet and resulting in a negatively charged O2- ion.
What do metals like sodium (Na) and magnesium (Mg) have in common regarding ion formation?
-Both sodium and magnesium are metals that lose their valence electrons to form cations. Group 1 metals like Na lose one electron to form +1 charged ions, while Group 2 metals like Mg lose two electrons to form +2 charged ions.
How do non-metals like oxygen (O) and fluorine (F) form ions and what are their charges?
-Non-metals like oxygen and fluorine gain electrons to form anions. Oxygen, with six valence electrons, gains two electrons to form an O2- ion with a -2 charge. Fluorine, with seven valence electrons, gains one electron to form an F- ion with a -1 charge.
What is a common characteristic of the ions formed by the gain or loss of electrons?
-The ions formed by the gain or loss of electrons have the same electronic structure as their nearest noble gas, achieving a stable configuration.
How do the elements in Group 3 and Group 5 differ in their ion formation?
-Group 3 elements, like aluminium, lose three electrons to form ions with a +3 charge, while Group 5 elements, like nitrogen, gain three electrons to form ions with a -3 charge.
What is a notable characteristic of Group 4 elements, such as carbon, when forming compounds?
-Group 4 elements, such as carbon, tend to form covalent compounds rather than ionic ones, as they typically share electrons to achieve a stable electronic configuration.
Outlines
🔬 Understanding Ions and Their Formation
This paragraph introduces the concept of ions, explaining that they are charged particles formed when an atom gains or loses electrons. Electrons, being negatively charged, result in a positive charge when an atom loses them, creating a cation. Conversely, a negative charge is produced when an atom gains electrons, forming an anion. The paragraph uses the mnemonic 'cats have paws' to remember cations are positively charged. It provides examples of sodium (Na) and oxygen (O) forming ions, and emphasizes that ions have a complete outer shell similar to noble gases. The paragraph concludes with an invitation to practice drawing the electronic structure of a calcium (Ca) atom and to consider the commonalities between sodium and magnesium (Mg) as metals that form cations.
Mindmap
Keywords
💡Ions
💡Electrons
💡Charge
💡Cations
💡Anions
💡Valence Electrons
💡Noble Gases
💡Metals
💡Non-metals
💡Electronic Structure
💡Chemical Formula
Highlights
An ion is a charged particle formed when an atom gains or loses electrons.
Electrons are negatively charged, so the gain or loss of electrons creates a charge on the atom.
Positively charged ions are called cations, while negatively charged ions are called anions.
An easy way to remember is that 'cats have paws', associating 'cations' with positively charged ions.
Sodium (Na) loses its one valence electron to become a positively charged Na+ ion.
The charge of an ion is written as a superscript when writing the formula.
The electronic structure of an ion is enclosed in square brackets with the charge written at the top right corner.
Oxygen (O) gains two electrons to form a negatively charged O2- ion.
Ions have a complete outer shell with the same electronic structure as a noble gas.
Metals, such as Na and Mg, lose their valence electrons to form cations.
Group 1 metals like Na have one valence electron and form +1 charged ions.
Group 2 metals like Mg have two valence electrons and form +2 charged ions.
Non-metals, such as O and F, tend to gain electrons to form anions.
Oxygen, being in Group 6, gains two electrons to form an O2- ion with a -2 charge.
Fluorine, in Group 7, gains one electron to form an F- ion with a -1 charge.
Group 3 elements like aluminium lose three electrons to form +3 charged ions.
Group 5 elements like nitrogen gain three electrons to form -3 charged ions.
Group 4 elements, such as carbon, tend to form covalent compounds.
The conclusion emphasizes that ions have the same electronic structure as their nearest noble gas.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: