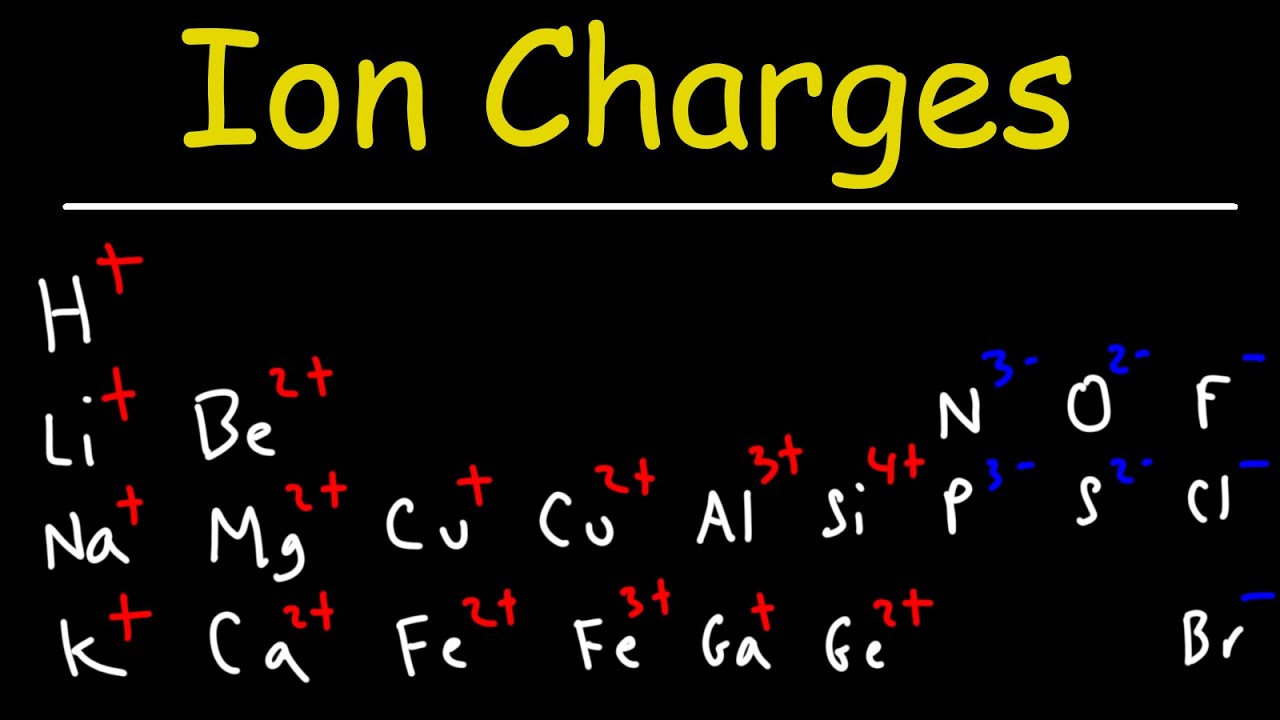ALEKS: Deducing the ions in a polyatomic ionic compound from its empirical formula
TLDRThis educational video script guides viewers on how to deduce the cations and anions in polyatomic ionic compounds from their empirical formulas. It emphasizes the importance of having a periodic table and a table of common polyatomic ions for reference. The script explains the use of parentheses to identify polyatomic ions and provides a systematic approach to determine the charge of ions, including those with multiple possible charges. The method involves using the periodic table, total charge balance, and the presence of multiple atom types to identify polyatomic ions and deduce their charges.
Takeaways
- 📚 To solve the problem of deducing ions in a polyatomic ionic compound, have a periodic table and a table of common polyatomic ions handy.
- 🔍 Parentheses in a compound formula indicate the presence of a polyatomic ion, making it easier to identify.
- 🌐 Use a table of polyatomic ions to find the specific ion and its charge, such as NH4+ for ammonium.
- 🚫 Do not include parentheses or quantities in the ion formula when identifying cations and anions for the problem.
- 🧩 For compounds without parentheses, count the total types of atoms to determine if a polyatomic ion is present.
- 🔎 If a compound has three or more types of atoms, it likely contains a polyatomic ion, which can be either CRP or PO4 in the given example.
- 🔋 Check the periodic table to predict the charge of non-polyatomic ions, like sulfur forming a -2 ion.
- 🔄 Elements that form multiple ions with different charges require additional strategies to determine their charge in a compound.
- ⚖️ The total sum of charges of all ions in a compound must equal zero, which helps in deducing the charge of unknown ions.
- 📉 For elements like chromium, where the charge is not easily predictable, use the charge balance rule to determine the necessary charge.
- 🏷️ When no polyatomic ion is present, the first element in the formula is typically the cation and the second is the anion, with quantities indicated separately.
Q & A
What is the main topic of the video?
-The video is about teaching viewers how to deduce the ions in a polyatomic ionic compound from its empirical formula.
What tools are recommended to have while solving the problem presented in the video?
-A periodic table and a table of common polyatomic ions are recommended, with a specific mention of a table from socratic.org.
What is the significance of parentheses in the formulas of ionic compounds?
-Parentheses in a formula indicate that the contents within them represent a polyatomic ion.
How does the video suggest determining the charge of the ammonium ion (NH4)?
-The video suggests looking up the NH4 ion in a table of polyatomic ions to find its charge, which is +1.
What is a trick mentioned in the video for predicting the charge of an ion based on its position in the periodic table?
-The trick involves a pattern of charges across columns: +1, +2, +3, skip a column, -3, -2, -1, skip a column. This helps predict the charge of elements in certain columns.
How does the video approach determining the charge of a non-polyatomic ion like sulfide?
-The video suggests using the periodic table and the trick mentioned to predict the charge of sulfur, which is -2 for the sulfide ion.
What is the method for identifying a polyatomic ion when parentheses are not present?
-Count the total number of different types of atoms in the compound; if there are three or more, it likely contains a polyatomic ion.
How does the video explain determining the charge of chromium in a compound without a predictable charge?
-By using the fact that the sum of charges in a compound must equal zero, and knowing the charge of the phosphate ion (-3), the video deduces that chromium must have a +3 charge.
What is the rule for identifying the cation and anion in a compound without a polyatomic ion?
-In the absence of a polyatomic ion, the first element in the formula is the cation, and the second is the anion.
How does the video handle the situation when the charge of an ion cannot be easily predicted from the periodic table?
-The video uses the rule that the total charge of all ions in a compound must sum to zero to deduce the charge of the unpredictable ion.
What does the video suggest when you cannot find an ion in a table of polyatomic ions?
-If an ion like bromine is not found in the table of polyatomic ions, it indicates that the ion is not part of a polyatomic ion and should be considered individually with its predictable charge.
Outlines
🔍 Identifying Polyatomic Ions in Ionic Compounds
This paragraph explains the process of identifying polyatomic ions within ionic compounds using the empirical formula. It emphasizes the importance of having a periodic table and a table of common polyatomic ions. The video script demonstrates how to deduce the presence of polyatomic ions by looking for parentheses in the formula, which enclose the polyatomic ion. The example of ammonium sulfide is used to illustrate the process of identifying the cation (NH4+) and anion (sulfide), and determining their charges using the periodic table and polyatomic ion table. The paragraph also introduces a trick for predicting the charges of certain elements based on their position in the periodic table.
📚 Deciphering Ionic Compounds Without Parentheses
The second paragraph delves into how to approach ionic compounds without parentheses to identify polyatomic ions. It suggests counting the total types of atoms to determine if a polyatomic ion is present. If there are three or more types, the polyatomic ion stays together in the compound. The example of chromium phosphate is used to illustrate this process, where the polyatomic ion is identified as PO4 (phosphate) with a -3 charge. The paragraph also discusses how to determine the charge of the cation, in this case, chromium, by using the principle that the sum of charges in a compound must equal zero. The video script guides viewers through the process of identifying the cation and anion in various compounds, including those with multiple possible charges for the cation, by ensuring the total charge neutrality of the compound.
Mindmap
Keywords
💡Polyatomic Ionic Compound
💡Empirical Formula
💡Periodic Table
💡Polyatomic Ion
💡Charge
💡Parentheses
💡Cation
💡Anion
💡Neutral Compound
💡Chromium
💡Bromide
Highlights
Introduction to solving the Alex problem of deducing ions in a polyatomic ionic compound from its empirical formula.
The necessity of having a periodic table and a table of common polyatomic ions for solving the problem.
The use of parentheses in formulas to identify polyatomic ions.
Identifying NH4 as a polyatomic ion with a positive charge from a table of polyatomic ions.
Instructions on not including the quantity of ions when writing the formula of ions.
How to determine the charge of non-polyatomic ions using the periodic table and a memorized trick.
The method to predict the charge of sulfur ions based on their position in the periodic table.
The strategy for identifying polyatomic ions when there are no parentheses present by counting the types of atoms.
The deduction that the polyatomic ion in the compound is either CRP or PO4 based on the types of atoms.
Using a table of polyatomic ions to confirm the presence of the PO4 ion and its charge.
Determining the charge of chromium ions by balancing the total charge to zero in the compound.
The importance of not confusing the quantity of ions with the charge of the ions when solving the problem.
Identifying the presence of a polyatomic ion in a compound with three different types of atoms.
The identification of SO4 as a polyatomic ion with a charge of -2 and the deduction of the copper ion's charge.
The absence of a polyatomic ion in a compound with only two types of atoms and the convention for cations and anions.
The method to determine the charge of vanadium and bromide ions when there is no polyatomic ion present.
The final deduction that vanadium must have a +3 charge to balance the -1 charges of three bromide ions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: