Writing Algebraic Equations in Chemistry
TLDRThe video script offers an insightful guide on writing algebraic equations in chemistry, focusing on two main topics: redox reactions and isotopes. It explains the importance of understanding oxidation numbers, which represent the loss or gain of electrons in a chemical reaction, and outlines specific rules to determine these numbers. The video demonstrates how to apply these rules systematically and use algebraic equations to find missing oxidation numbers, ensuring they sum up to the compound's total charge. Additionally, the script covers calculating the percent abundance of isotopes, showing how to set up and solve algebraic equations to find the mass and percentage of each isotope. The presenter encourages practice with a variety of problems available on their website, melissa.help/practice, to solidify understanding and prepare for exams.
Takeaways
- 🔢 **Writing Algebraic Equations**: In chemistry, writing algebraic equations is crucial for solving problems related to redox reactions and isotopes.
- ⚛️ **Oxidation Numbers**: Oxidation numbers are used to determine electron transfer and to identify the species undergoing oxidation or reduction.
- 📜 **Oxidation Number Rules**: There are specific rules to assign oxidation numbers which should be applied in a given order, starting with the element in its solid state having zero oxidation number.
- 💡 **Applying Rules**: The sum of oxidation numbers for all elements in a compound must equal the total charge of the compound; this can be used as a check for correctness.
- 🧩 **Solving for Unknowns**: When oxidation numbers for all elements except one are known, an algebraic equation can be set up to solve for the unknown value.
- 📝 **Example Calculation**: For a compound with known oxidation numbers for hydrogen and oxygen, but an unknown for chlorine, setting up an equation can reveal the oxidation number for chlorine is +5.
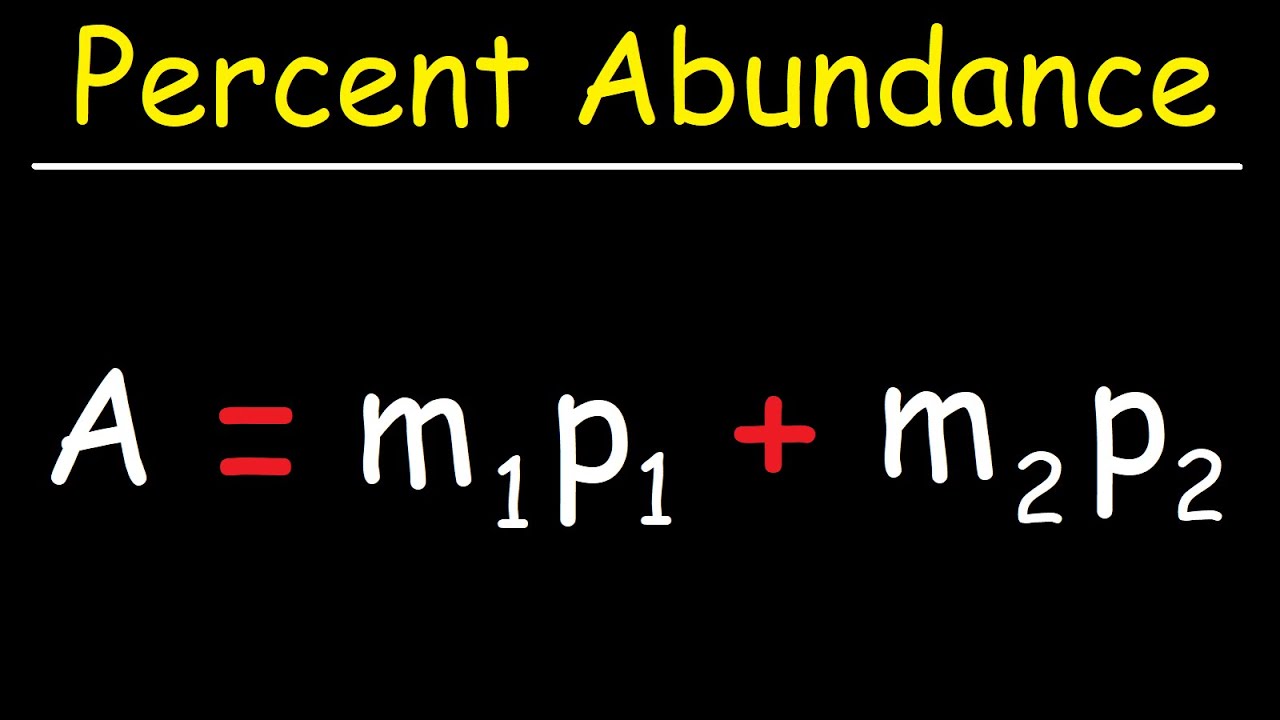
- 🔍 **Isotope Abundance**: Percent abundance of isotopes can be calculated using the average atomic mass and the masses of individual isotopes.
- 🔄 **Percent Abundance Formula**: The formula involves summing the product of mass and percent abundance for each isotope to get the average atomic mass.
- 🔢 **Algebraic Approach**: When calculating percent abundance, algebraic equations are used to express one unknown in terms of another, considering the total percent must equal 100%.
- 📊 **Combining Terms**: Like terms are combined, and algebraic manipulations are performed to solve for the unknowns, such as the percent abundances of isotopes.
- 🔗 **Additional Resources**: For practice problems, Melissa provides resources on her website to help solidify understanding and application of these concepts.
Q & A
What are the two main chemistry topics discussed in the transcript that require knowledge of algebraic equations?
-The two main chemistry topics discussed are redox reactions and isotopes.
What is the significance of oxidation numbers in redox reactions?
-Oxidation numbers help determine if electrons are being lost or gained and which reactant undergoes oxidation or reduction.
What is the first rule of oxidation numbers mentioned in the transcript?
-The first rule is that when an element is in its solid state, the oxidation number is zero.
How does the sum of oxidation numbers relate to the total charge of a compound?
-The sum of all oxidation numbers for all elements in a compound must add up to the total charge of the compound, molecule, or ion.
What is the oxidation number of hydrogen within a compound according to rule four?
-According to rule four, the oxidation number of hydrogen within a compound is positive 1.
How is the percent abundance of an isotope calculated?
-The percent abundance is calculated using the formula where the average atomic mass of all isotopes is set equal to the sum of each individual isotope's mass multiplied by its percent abundance.
What is the purpose of writing algebraic equations when determining the oxidation numbers of elements in a compound?
-Algebraic equations are used to find the unknown oxidation numbers of elements in a compound when the oxidation numbers of other elements are known.
Why is it necessary to consider the order of the oxidation number rules when applying them?
-The order is important because it ensures that the rules are applied correctly and consistently, which is necessary for accurately determining oxidation numbers.
How many oxygens are considered in the second example provided in the transcript?
-In the second example, there are seven oxygens considered.
What is the oxidation number for chlorine in the compound discussed in the transcript?
-The oxidation number for chlorine in the compound is positive 5.
How can one represent the percent abundance of the second isotope in terms of the first isotope's percent abundance?
-The percent abundance of the second isotope can be represented as (1 - x), where x is the percent abundance of the first isotope.
What is the website mentioned in the transcript for additional practice problems?
-The website mentioned for additional practice problems is melissa.help/practice.
Outlines
🔢 Understanding Algebraic Equations in Chemistry
This paragraph focuses on the importance of writing algebraic equations in chemistry, particularly for redox reactions and isotopes. It explains the concept of oxidation numbers and their role in determining electron transfer. The paragraph outlines specific rules for assigning oxidation numbers to elements in various states and compounds, emphasizing the sequence in which these rules should be applied. It also highlights the necessity of ensuring that the sum of oxidation numbers equals the total charge of the compound, molecule, or ion. An example is provided to illustrate how to use these rules and solve for unknown oxidation numbers algebraically, demonstrating the process step by step.
🧬 Calculating Oxidation Numbers and Isotope Abundance
The second paragraph delves into another application of algebraic equations in chemistry: calculating oxidation numbers and the percent abundance of isotopes. It demonstrates how to find the oxidation numbers for elements in a compound when not all are known, using algebraic methods to solve for the missing values. The paragraph also introduces the concept of percent abundance of isotopes and provides a formula for calculating it. An example is given to show how to use the formula and algebraic manipulation to find the percent abundance of different isotopes based on their masses and the element's average atomic weight. The paragraph concludes with a resource for additional practice problems on the topic.
Mindmap
Keywords
💡Algebraic Equations
💡Redox Reactions
💡Isotopes
💡Oxidation Numbers
💡Percent Abundance
💡Periodic Table
💡Diatomic Molecules
💡Halogens
💡Charge of a Compound
💡Average Atomic Mass
💡Practice Problems
Highlights
Algebraic equations are essential in chemistry for solving problems related to redox reactions and isotopes.
Oxidation numbers help determine electron transfer and identify which reactant undergoes oxidation or reduction.
There are specific oxidation number rules to memorize, which are applied in a particular order.
Rule one states that the oxidation number of an element in its solid state is zero.
Diatomic molecules have an oxidation number of zero, excluding certain exceptions like oxygen.
An element with a charge has an oxidation number equal to that charge.
Hydrogen within a compound has an oxidation number of +1.
Elements in group 1 of the periodic table have an oxidation number of +1 when in a compound.
Elements in group 2 have an oxidation number of +2 within a compound.
Oxygen within a compound typically has an oxidation number of -2.
Halogens within a compound have an oxidation number of -1.
The sum of all oxidation numbers must equal the total charge of the compound, molecule, or ion.
Writing algebraic equations is a method to find missing oxidation numbers, such as for chlorine in a compound.
The oxidation number for chlorine in a compound can be determined by solving an algebraic equation.
Percent abundance of isotopes is calculated using the average atomic mass and individual isotope masses and abundances.
To find the percent abundance of isotopes, algebraic equations can be used to express one variable in terms of another.
The algebraic equation for percent abundance involves the sum of the mass and percent abundance of each isotope.
Practice problems for understanding oxidation numbers and isotope percent abundance are available on melissa.help/practice.
Transcripts
Browse More Related Video

Oxidation, Reduction, and Redox Balancing Redox Reactions

How to Find Oxidation Numbers (Rules and Examples)

How to Calculate Oxidation Numbers Introduction

Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry

Redox Reactions: Crash Course Chemistry #10
How To Find The Percent Abundance of Each Isotope - Chemistry
5.0 / 5 (0 votes)
Thanks for rating: