How To Find The Percent Abundance of Each Isotope - Chemistry
TLDRThis video tutorial explains the method to calculate the percent abundance of isotopes within an element, using bromine as an example. It demonstrates how to use the given atomic masses of isotopes and the average atomic mass to determine the relative proportions of each isotope on Earth. The process involves setting up an algebraic equation and solving for the unknown percent abundances, ultimately revealing that bromine-79 and bromine-81 have approximately equal percentages, with bromine-79 being slightly more abundant.
Takeaways
- 📊 The video explains the method to calculate the percent abundance of isotopes in an element, using bromine as an example.
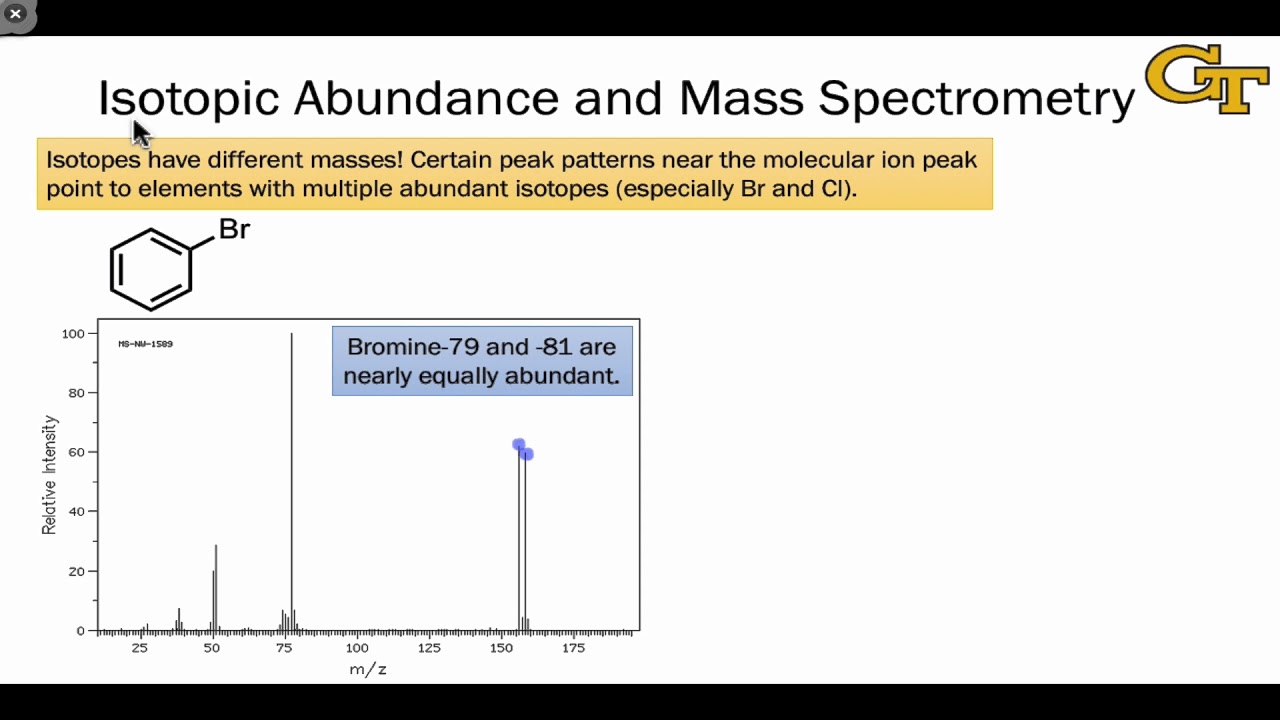
- 🔬 Bromine consists of two predominant naturally occurring isotopes, with atomic masses of 79 and 81.
- 📈 The weighted average atomic mass of bromine is approximately 79.9 atomic mass units.
- 🤔 The percent abundance can be estimated by the position of the weighted average atomic mass between the isotopic masses.
- 📝 The calculation involves setting up an equation where the average atomic mass equals the product of the mass of each isotope and its percent abundance.
- 🎯 The symbol 'a' is used to represent the average atomic mass in the calculation.
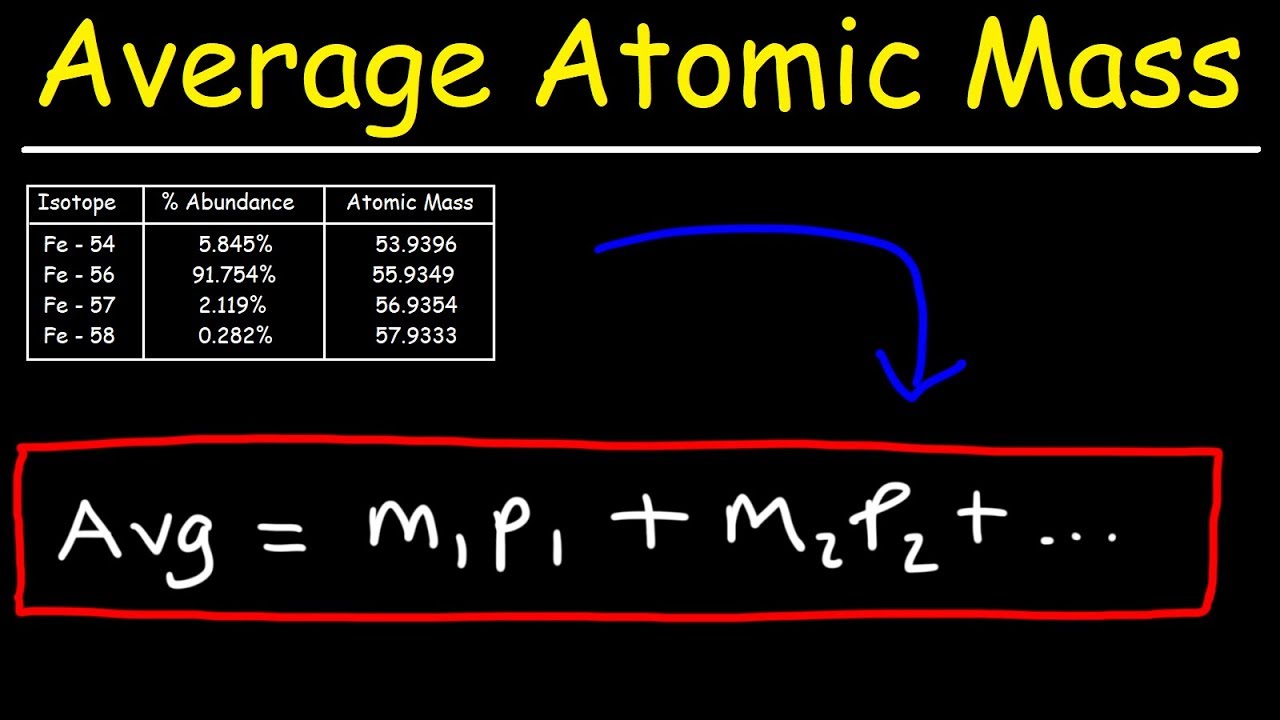
- 🔢 The equation for calculating percent abundance is m1 * p1 + m2 * p2 = a, where m1 and m2 are the masses of isotopes and p1 and p2 are their respective percent abundances.
- 🧠 The process includes solving an algebraic equation to find the exact percent abundance of each isotope.
- 📌 The calculated percent abundance for bromine 79 is approximately 50.9%, and for bromine 81, it is 49.1%.
- 🔄 The video suggests checking the work by plugging the calculated percent abundances back into the formula to ensure the result matches the given average atomic mass.
- 🎓 The video is part of a broader series on chemistry and other subjects like physics, algebra, and calculus available on the tutor's website and YouTube channel.
Q & A
What is the topic of the video?
-The video discusses how to calculate the percent abundance of isotopes that make up a specific element, using bromine as an example.
How many naturally occurring isotopes does bromine have?
-Bromine consists of two predominant naturally occurring isotopes, although there are others.
What are the atomic masses of the two isotopes of bromine mentioned in the video?
-The atomic masses of the two isotopes are 79 for bromine 79 and 81 for bromine 81.
What is the weighted average atomic mass of bromine given in the video?
-The weighted average atomic mass of bromine is 79.9 atomic mass units.
What is the process to calculate the percent abundance of isotopes?
-The process involves using the formula where the average atomic mass is equal to the mass of the isotope times its percent abundance, summed for all isotopes.
What are the mass values used for bromine 79 and bromine 81 in the calculation?
-For bromine 79, the mass value used is 78.918, and for bromine 81, it is 80.916.
How does the video estimate the percent abundance of bromine 79 and bromine 81 before calculation?
-The video initially estimates that bromine 79 might have a 40% abundance and bromine 81 a 60% abundance, based on the average atomic mass being close to the midpoint of the two isotopes' masses.
What is the actual calculated percent abundance of bromine 79 and bromine 81?
-The calculated percent abundance is approximately 50.9% for bromine 79 and 49.1% for bromine 81.
How can you verify the correctness of the calculated percent abundance values?
-You can verify the values by plugging them back into the formula for the average atomic mass and checking if the result matches the given average atomic mass of 79.9 atomic mass units.
What is the significance of the average atomic mass being close to the midpoint of the isotopes' masses?
-If the average atomic mass is close to the midpoint, it suggests that the element's isotopic composition is approximately equal, or a 50-50 mix of the isotopes.
Where can viewers find more educational content related to chemistry and other subjects?
-Viewers can find more educational content on the video creator's channel or website, www.thevideodashtutor.net, which includes playlists on chemistry, physics, algebra, trigonometry, pre-calculus, and calculus.
Outlines
📊 Calculating Isotope Percent Abundance
This paragraph introduces the concept of calculating the percent abundance of isotopes for the element bromine. It explains that bromine consists of two predominant naturally occurring isotopes and provides the atomic masses of bromine-79 and bromine-81. The goal is to determine the relative percent abundance of each isotope on Earth, given the average atomic mass of bromine is 79.9. The paragraph outlines the process of using a weighted average formula to calculate the exact percent abundance, starting with the equation m1*p1 + m2*p2, where m1 and m2 are the masses of the isotopes and p1 and p2 are their respective percent abundances.
🧮 Solving for Isotope Abundance Percentages
The paragraph details the algebraic process of solving for the percent abundance of bromine-79 (p1) and bromine-81 (p2). It begins by assuming hypothetical values for p1 and p2 to illustrate the steps, then proceeds to solve the equation using the given average atomic mass. The calculation involves distributing and combining like terms, and isolating the variable x, which represents the percent abundance of bromine-79. The result yields a 50.9% abundance for bromine-79 and a 49.1% abundance for bromine-81, confirming the initial hypothesis that the abundances are approximately equal, with a slight preference for bromine-79 due to the average atomic mass being closer to 79.9 than to 81.
🎓 Summary and Additional Resources
In the final paragraph, the video concludes by summarizing the method for finding the percent abundance of isotopes in an element, emphasizing that the process demonstrated is applicable to any element with two isotopes. The speaker also promotes additional chemistry videos and other educational content on their channel and website, inviting viewers to explore resources on various subjects such as physics, algebra, trigonometry, pre-calculus, and calculus.
Mindmap
Keywords
💡Percent Abundance
💡Isotopes
💡Atomic Mass
💡Weighted Average
💡Bromine 79
💡Bromine 81
💡Algebra
💡Chemistry
💡Element
💡Natural Occurrence
💡Video Tutorial
Highlights
The video explains how to calculate the percent abundance of isotopes that make up an element, specifically using the element bromine as an example.
Bromine consists of two predominant naturally occurring isotopes, with others present but in smaller amounts.
The atomic mass of bromine is given as 79 and 81, with a weighted average atomic mass of 79.9.
The goal is to determine the relative percent abundance of bromine-79 and bromine-81 on Earth.
The weighted average atomic mass (79.9) suggests an almost 50-50 distribution between the two isotopes.
The process involves using algebra to set up an equation with the mass of each isotope multiplied by its unknown percent abundance.
The equation for bromine-79 (Br79) is set up as m1 * p1, where m1 is the mass of Br79 and p1 is its percent abundance.
For bromine-81 (Br81), the equation is m2 * p2, with m2 being the mass of Br81 and p2 being its percent abundance.
The percent abundance for Br81 is represented as 1 - x, assuming Br79 has an initial assumed percent abundance of 40%.
By solving the equation, we find that the actual percent abundance of Br79 is 50.9% and Br81 is 49.1%.
The calculated percent abundances are approximately equal, as the average atomic mass (79.9) is close to the midpoint of the two isotopes' masses.
There is slightly more bromine-79 than bromine-81 because the average atomic mass is closer to 79.
The method demonstrated can be used to find the percent abundance of isotopes for any element with multiple isotopes.
The video also provides a way to check the work by plugging the calculated percent abundances back into the formula to ensure they yield the average atomic mass.
The video is part of a series on chemistry and is available on the channel, with additional playlists on physics, algebra, trigonometry, pre-calculus, and calculus.
The video includes a website link (www.thevideodashtutor.net) for viewers to access more educational content.
The video concludes by encouraging viewers to like, comment, and subscribe for more content.
Transcripts
Browse More Related Video

Atomic Mass: How to Calculate Isotope Abundance
13.04 Isotopic Abundance in Mass Spectrometry

Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry
How To Calculate The Average Atomic Mass
Protons Neutrons Electrons Isotopes - Average Mass Number & Atomic Structure - Atoms vs Ions

What's the Difference between Mass Number and Atomic Mass?
5.0 / 5 (0 votes)
Thanks for rating: