Isotopes, Percent Abundance, Atomic Mass | How to Pass Chemistry
TLDRThe video script by Melissa Maribel offers a clear explanation of isotopes and how to calculate their percent abundance. Using relatable examples, Melissa simplifies the concept of isotopes as different versions of the same element with varying masses due to different neutron counts. She demonstrates the calculation of an element's average atomic mass based on the abundance and mass of its isotopes, and also shows how to determine the percent abundance of isotopes given an element's average atomic mass. The script is an educational guide designed to help students understand these chemistry topics and prepare for exams.
Takeaways
- 🌟 Isotopes are different versions of the same element with the same atomic number but different masses due to varying numbers of neutrons.
- 📚 The concept of isotopes is introduced using a personal weight example to illustrate the idea of 'different versions' of the same thing.
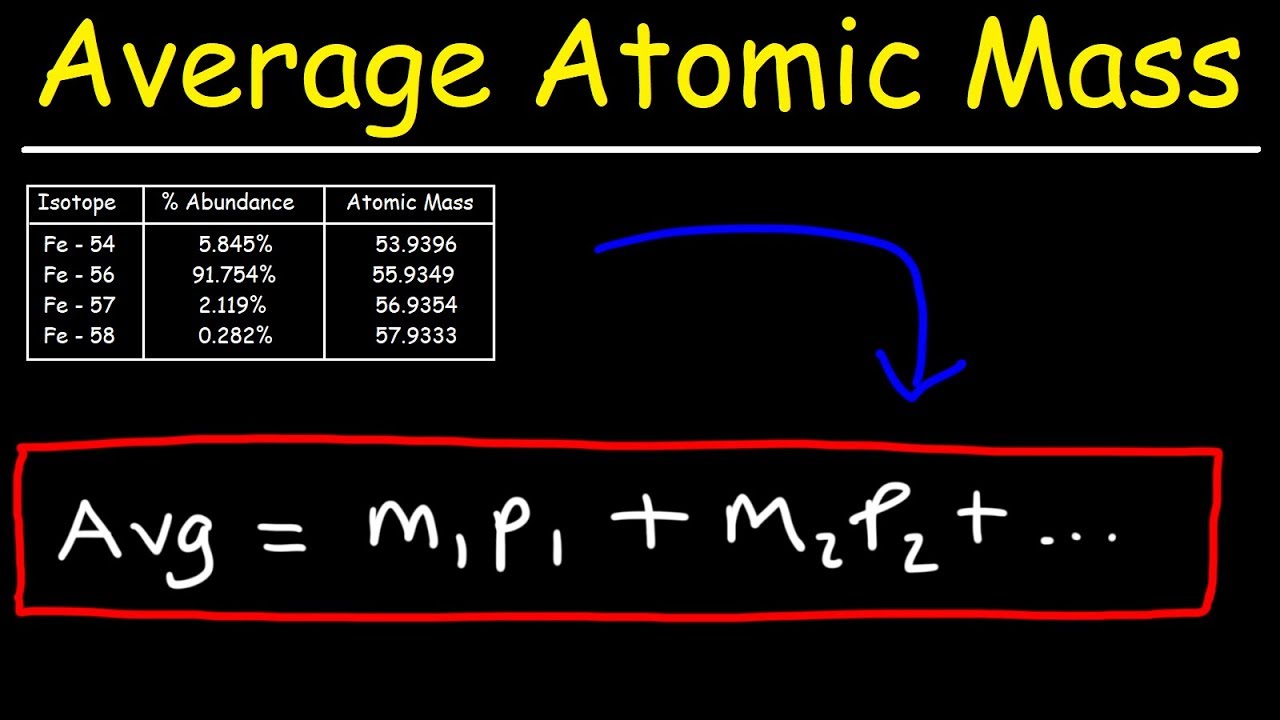
- 📊 The atomic mass listed on the periodic table is the average mass of all isotopes of an element, calculated based on their percent abundance.
- 🔢 Percent abundance is the percentage of a particular isotope relative to the total amount of the element, and it's used to calculate the average atomic mass.
- ✂️ To find the average atomic mass, convert percentages to decimals, multiply by the mass of each isotope, and sum the results.
- 📐 The example demonstrates calculating the average atomic mass of an element X with four isotopes, resulting in 87.62 amu.
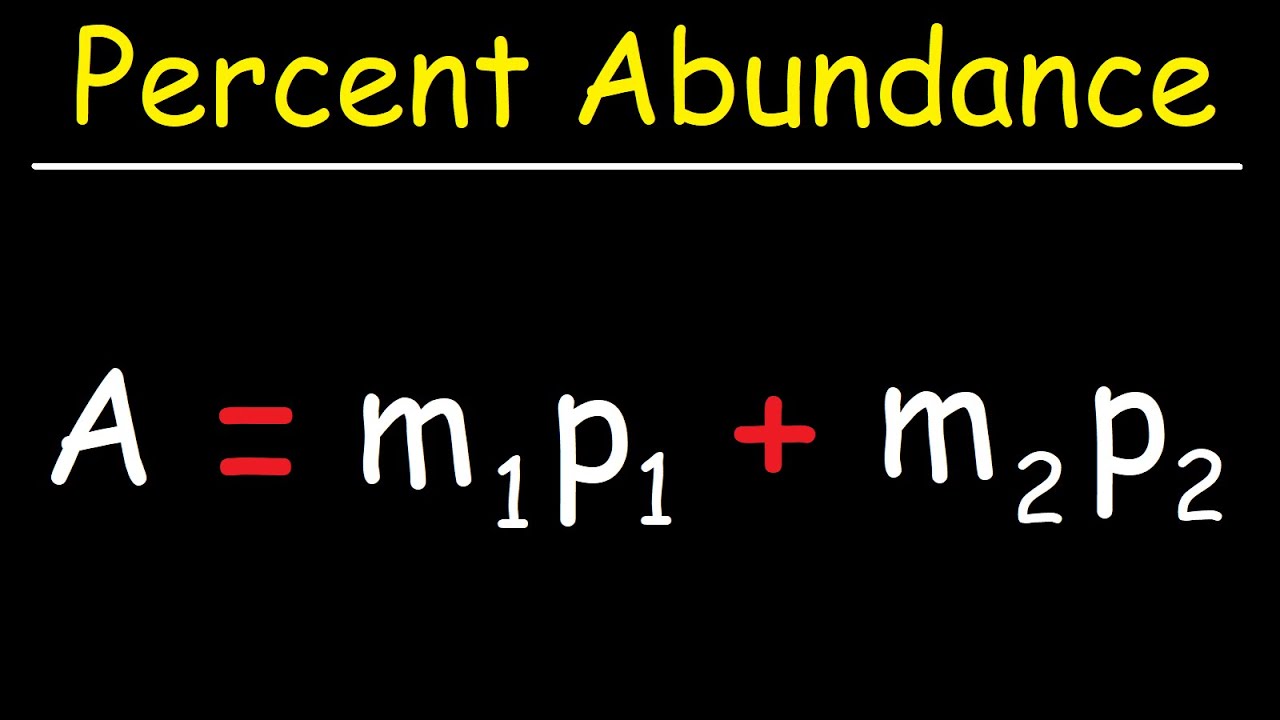
- 🧬 The second example involves determining the percent abundance of copper isotopes given their masses and the average atomic mass of copper.
- 📘 The percent abundance formula is used to solve for unknown percentages of isotopes, setting up an equation and solving for 'X'.
- 📝 The solution process involves algebraic manipulation to isolate the variable representing the unknown percent abundance.
- 📊 The calculated percent abundances for copper isotopes are approximately 69.15% for ^63Cu and 30.85% for ^65Cu.
- 📚 The video script emphasizes the importance of practicing such problems as they frequently appear on exams.
- 🎓 The tutor offers additional help for students who feel unprepared, suggesting tutoring services for further assistance.
Q & A
What is an isotope?
-An isotope is a different version of the same element, having the same number of protons but different numbers of neutrons, resulting in different atomic masses.
How does the concept of isotopes relate to Melissa Maribel's personal example?
-Melissa Maribel uses her different weights at various years as an analogy to explain isotopes. Just as she is the same person despite different weights, isotopes are the same element with different atomic masses.
What is the chemical symbol for gold?
-The chemical symbol for gold is 'Au'.
What is the significance of the atomic mass listed on the periodic table?
-The atomic mass listed on the periodic table is the average mass of all the isotopes of that element.
What is percent abundance in the context of isotopes?
-Percent abundance refers to the proportion of each isotope in a naturally occurring element, expressed as a percentage of the total.
How is the average atomic mass of an element calculated?
-The average atomic mass of an element is calculated by multiplying the mass of each isotope by its respective percent abundance and then summing these products.
What is the purpose of converting percentages to decimals in the calculation of average atomic mass?
-Converting percentages to decimals simplifies the calculation process by allowing for direct multiplication with the isotope masses without dealing with percentages.
What is the significance of significant figures in scientific measurements?
-Significant figures are the digits in a number that carry meaning contributing to its precision. They indicate the reliability of the data and are used to round the final result to match the given data's precision.
What is the average atomic mass of copper given in the script?
-The average atomic mass of copper given in the script is 63.546 amu.
How is the percent abundance of isotopes calculated when the average atomic mass is known?
-The percent abundance is calculated by setting up an equation where the sum of the products of the isotope masses and their respective unknown abundances equals the average atomic mass, and then solving for the unknown abundances.
What are the two isotopes of copper mentioned in the script and their respective masses?
-The two isotopes of copper mentioned are copper-63 with a mass of 62.9296 amu and copper-65 with a mass of 64.9278 amu.
What are the calculated percent compositions of the two copper isotopes in the script?
-The calculated percent compositions are 69.15% for copper-63 and 30.85% for copper-65.
Outlines
🔬 Understanding Isotopes and Calculating Percent Abundance
This paragraph introduces the concept of isotopes using a personal weight analogy and delves into the chemistry behind them. Melissa Maribel, the host, explains that isotopes are different forms of the same element with varying mass due to different numbers of neutrons, but the same atomic number. She uses gold as an example, illustrating three isotopes with different atomic mass units (amu). The paragraph also covers the calculation of average atomic mass for an element with multiple isotopes, emphasizing the importance of percent abundance and how it's used to find the average atomic mass by converting percentages to decimals and multiplying them by the mass of each isotope. The process is demonstrated with an example involving four isotopes of an element X, resulting in an average atomic mass of 87.62 amu.
📊 Determining Percent Abundance of Copper Isotopes
In this segment, the focus shifts to calculating the percent abundance of copper isotopes, given their masses and the average atomic mass of copper. The example provided involves two isotopes of copper, Cu-63 with a mass of 62.9296 amu and Cu-65 with a mass of 64.9278 amu, and the task is to find their respective percent abundances based on the average atomic mass of 63.546 amu. The process involves setting up an equation where the sum of the products of the mass of each isotope and its unknown percent abundance (represented as X for the first isotope and 1-X for the second) equals the average atomic mass. Solving this equation yields the percent abundances: 69.15% for Cu-63 and 30.85% for Cu-65. This demonstrates how to apply the percent abundance formula to determine the composition of elements based on their isotopes.
📚 Preparing for Exams with Practice Problems on Isotopes
The final paragraph serves as a motivational call to action for students, urging them to practice problems related to isotopes, as they are common exam questions. The tutor emphasizes the importance of preparation and offers tutoring services for those who need additional help. She encourages students to check the description box for available tutoring times and ends with a friendly reminder to like, subscribe, and look forward to the next video. This paragraph reinforces the practical application of the concepts learned and provides a resource for further learning and support.
Mindmap
Keywords
💡Isotope
💡Percent Abundance
💡Atomic Mass Unit (amu)
💡Average Atomic Mass
💡Significant Figures
💡Percent Composition
💡Isotope Notation
💡Element X
💡Tutoring
💡Practice Problems
Highlights
Introduction to the concept of isotopes and their calculation of percent abundance.
Isotopes are different versions of the same element with the same atomic number but different masses and neutrons.
The atomic mass listed on the periodic table is the average of all isotopes of an element.
Explanation of percent abundance and its role in calculating the average atomic mass of an element.
Conversion of percentages to decimals for calculation purposes.
The formula for calculating the average atomic mass using percent abundance and mass of isotopes.
Example calculation of the average atomic mass of an element X with four isotopes.
The importance of rounding to significant figures in scientific calculations.
The second example involves solving for percent composition or percent abundance of copper isotopes.
Given data on copper isotopes and their masses, and the task to find their percent abundance.
Use of the percent abundance formula to determine the percentage of each isotope in copper.
Method to find the unknown percent abundance by setting up an equation with the known average atomic mass.
Solving the equation to find the percent abundance of the first copper isotope.
Calculation of the second isotope's percent abundance using the complement to 100%.
Final determination of the percent compositions for both copper isotopes: 69.15% for Cu-63 and 30.85% for Cu-65.
Encouragement for students to practice problems similar to those on exams for better preparation.
Offer of tutoring services for students who need additional help with understanding isotopes and related calculations.
Transcripts
Browse More Related Video

GCSE Chemistry - Elements, Isotopes & Relative Atomic Mass #2
How To Calculate The Average Atomic Mass

Atomic Mass: How to Calculate Isotope Abundance
How To Find The Percent Abundance of Each Isotope - Chemistry
Protons Neutrons Electrons Isotopes - Average Mass Number & Atomic Structure - Atoms vs Ions

Atomic Mass: Introduction
5.0 / 5 (0 votes)
Thanks for rating: