How to Find Limiting Reactants | How to Pass Chemistry
TLDRThe video script explains the concept of limiting reactant through a strawberry smoothie analogy, demonstrating how to identify the reactant that will be used up first in a chemical reaction. It then applies this concept to a specific chemical reaction involving nitrogen and hydrogen gases to produce ammonia, guiding viewers through the steps of converting grams of reactants to grams of product using molar masses and mole ratios from a balanced equation. The video emphasizes the importance of understanding limiting reactants in chemical reactions and encourages practice for better comprehension.
Takeaways
- 🍓 The concept of limiting reactant is introduced through a strawberry smoothie analogy, where the reactants are strawberries and milk, and the product is the smoothie.
- 🥛 A limiting reactant is the ingredient that gets used up the fastest in a reaction and produces the least amount of product.
- 📜 In the example with N2 and H2 reacting to form NH3, the balanced chemical equation is key to determining the limiting reactant.
- 📊 To find the limiting reactant, convert grams of each reactant to grams of product using molar mass and mole-to-mole ratio from the balanced equation.
- 🔄 For N2, 14.32g gives way to calculating moles of N2, using the molar mass of 28.02g/mol, and then using the mole ratio to find moles of NH3.
- 🔄 Similarly, for H2, 4.21g is converted to moles of H2 using its molar mass, and then the mole ratio from the balanced equation is used to find moles of NH3.
- 📈 After converting moles of NH3 back to grams using the molar mass of NH3 (17.03g/mol), compare the theoretical yields from both reactants to determine the limiting reactant.
- 🏆 N2 is the limiting reactant in the given example because it produces the least amount of NH3 (17.42g) compared to H2 (23.68g).
- 📚 Detailed notes and examples for finding limiting reactants, excess reactants, theoretical yield, percent yield, and actual yield are available in the video description.
- 💪 Persistence and practice are emphasized as essential for mastering the process of determining limiting reactants and related chemical calculations.
Q & A
What is the concept of a limiting reactant?
-The limiting reactant is the ingredient or reactant in a chemical reaction that is used up the fastest and produces the least amount of product. It determines the maximum amount of product that can be formed in a chemical reaction.
How can you identify the limiting reactant in a chemical reaction?
-To identify the limiting reactant, you need to compare the amounts of reactants used in the reaction based on their stoichiometric ratios. The reactant that runs out first and produces the least amount of product is the limiting reactant.
What is the relationship between the reactants and the product in the strawberry smoothie example?
-In the strawberry smoothie example, strawberries and milk are the reactants, and the strawberry smoothie is the product. The recipe calls for one strawberry and one carton of milk to make one serving of the smoothie.
How many strawberry smoothies can be made with the given ingredients in the example?
-With four strawberries and two cartons of milk, and given that the recipe requires one strawberry and one carton of milk per serving, you can make two strawberry smoothies.
What is the excess reactant in the strawberry smoothie example?
-The excess reactant in the strawberry smoothie example is the ingredient that remains after making the smoothies. Since the recipe uses one strawberry and one carton of milk per serving, the excess reactant is the remaining strawberries after making two smoothies.
What is the theoretical yield in the context of the script?
-The theoretical yield refers to the maximum amount of product that can be produced from the limiting reactant. In the strawberry smoothie example, the theoretical yield is the number of smoothies that can be made using the limiting reactant, which is two smoothies.
How do you calculate the theoretical yield given the masses of reactants in a chemical reaction?
-To calculate the theoretical yield, you convert the grams of each reactant to grams of product using the stoichiometry from the balanced chemical equation. This involves converting grams to moles using molar masses, using the mole-to-mole ratios from the balanced equation, and then converting moles of product back to grams using the molar mass of the product.
What is the balanced equation for the production of NH3 in the given example?
-The balanced equation for the production of NH3 (ammonia) from N2 (nitrogen) and H2 (hydrogen) is not explicitly given in the script, but it can be inferred as N2 + 3H2 → 2NH3, based on the mole-to-mole ratios used in the example.
How much NH3 can be produced from 14.32g of N2 according to the script?
-From 14.32g of N2, 17.42g of NH3 can be produced as per the calculations provided in the script.
How much NH3 can be produced from 4.21g of H2 according to the script?
-From 4.21g of H2, 23.68g of NH3 can be produced as per the calculations provided in the script.
Which reactant is the limiting reactant for the production of NH3 in the given example?
-N2 is the limiting reactant for the production of NH3 in the given example because it produces the least amount of product (17.42g of NH3) compared to H2 (23.68g of NH3).
What additional resources are available for learning about limiting, excess reactants, and yields?
-The script mentions that detailed notes with examples covering limiting and excess reactants, theoretical yield, percent yield, actual yield, and all types of yields are available in a link provided in the description box.
Outlines
🥤 Introduction to Limiting Reactant with a Smoothie Analogy
This paragraph introduces the concept of limiting reactant using a relatable analogy of making a strawberry smoothie. It explains that the limiting reactant is the ingredient that gets used up the fastest in a chemical reaction, resulting in the least amount of product. The analogy uses strawberries and milk to illustrate this point, where milk is identified as the limiting reactant because it runs out faster than strawberries. The paragraph then transitions into a chemical reaction example involving nitrogen (N2) and hydrogen (H2) to produce ammonia (NH3), setting the stage for a step-by-step process to identify the limiting reactant.
📈 Calculation of Limiting Reactant in a Chemical Reaction
This paragraph delves into the step-by-step process of calculating the limiting reactant in a chemical reaction. It uses the given example of N2 and H2 reacting to form NH3 and provides a detailed explanation of how to convert grams of reactants to grams of product using molar masses and mole-to-mole ratios from the balanced chemical equation. The paragraph outlines the process of converting grams of N2 to grams of NH3 and then does the same for H2, ultimately comparing the amounts of NH3 that could be produced from each reactant to determine which one is the limiting reactant. The summary emphasizes the importance of understanding these calculations for grasping the concepts of theoretical yield and limiting reactant.
Mindmap
Keywords
💡Limiting Reactant
💡Strawberries
💡Cartons of Milk
💡Strawberry Smoothie
💡Theoretical Yield
💡Excess Reactant
💡Moles
💡Molar Mass
💡Balanced Equation
💡Conversion
💡Percent Yield
💡Actual Yield
Highlights
The introduction of the concept of limiting reactant using a strawberry smoothie analogy.
Strawberries represent one reactant with a limited quantity.
Milk represents another reactant, also in limited supply.
The smoothie represents the product of the reaction.
The limiting reactant is the one that gets used up the fastest.
The excess reactant is what remains after the reaction.
The theoretical yield is the maximum amount of product that can be produced.
A step-by-step process to determine the limiting reactant in a chemical reaction involving N2 and H2.
Conversion of grams of each reactant to grams of product using molar mass and mole-to-mole ratio.
The balanced chemical equation is crucial for determining mole-to-mole ratios.
The calculation of theoretical yield for N2 and H2 in the production of NH3.
Comparing the theoretical yields to identify the limiting reactant.
N2 is determined to be the limiting reactant based on the least amount of NH3 produced.
The importance of practice and persistence in understanding and applying the concept of limiting reactants.
The availability of detailed notes and examples for further understanding.
The encouragement for viewers to engage with the content and apply the knowledge.
The practical application of the concept in real-world scenarios, such as cooking.
The use of an analogy to simplify the understanding of a complex scientific concept.
Transcripts
Browse More Related Video

Introduction to Limiting Reactant and Excess Reactant
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
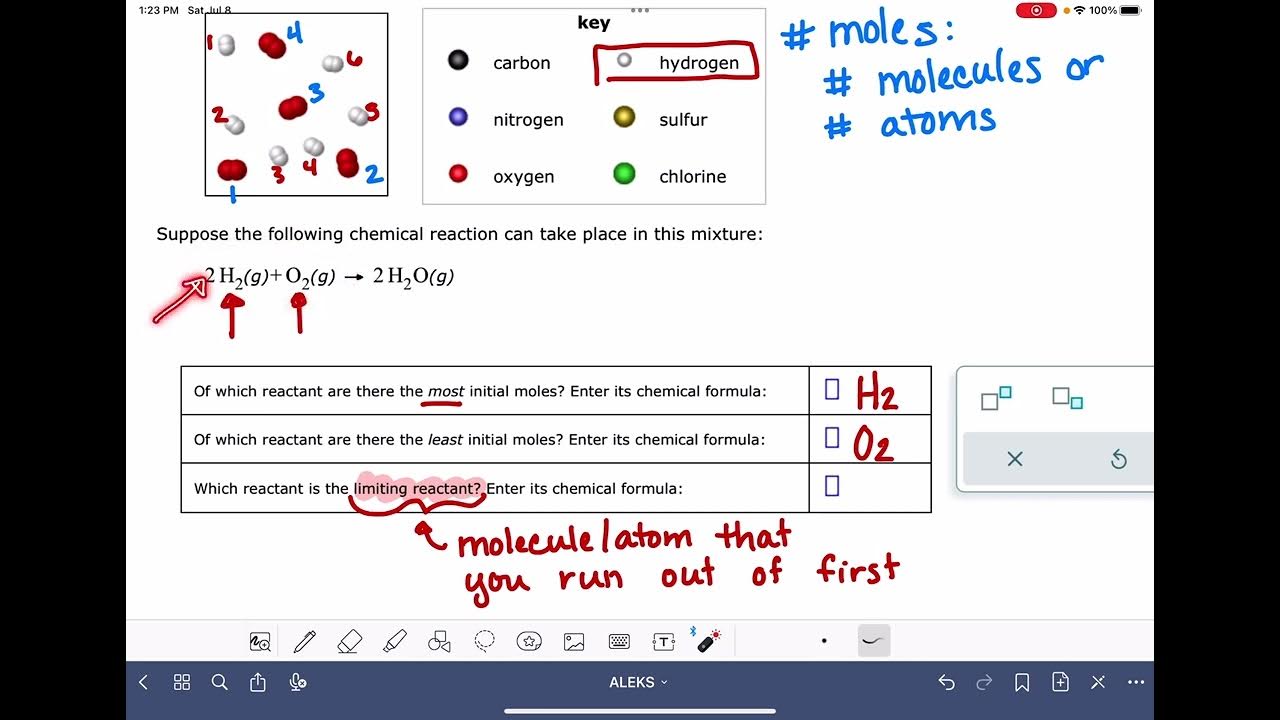
ALEKS: Identifying the limiting reactant in a drawing of a mixture

Limiting Reactant Practice Problems

Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
5.0 / 5 (0 votes)
Thanks for rating: